



生物多样性 ›› 2025, Vol. 33 ›› Issue (6): 24416. DOI: 10.17520/biods.2024416 cstr: 32101.14.biods.2024416
收稿日期:2024-09-18
接受日期:2025-03-11
出版日期:2025-06-20
发布日期:2025-07-28
通讯作者:
胡自民
基金资助:Received:2024-09-18
Accepted:2025-03-11
Online:2025-06-20
Published:2025-07-28
Contact:
Zimin Hu
Supported by:摘要:
基于谱系多样性特征推断冰期避难所的位置是分子系统地理学研究的重要内容之一, 这对于理解多样性的起源和进化模式以及全球气候变化背景下生物资源的保护和管理等具有重要意义。本文利用线粒体23S rRNA-tRNA-Val基因间区(intergenic spacer, IGS)和COX1对加拿大纽芬兰大浅滩(Grand Banks)的二裂墨角藻(Fucus distichus)种群开展了谱系多样性研究。通过比较北太平洋、西北大西洋和东北大西洋其他二裂墨角藻种群分子数据, 我们发现大浅滩种群的特有基因型数目、单倍型多样性(h = 0.6533)和核苷酸多样性(π = 0.0067)显著高于其他地区(h = 0.1487, π = 0.0022)。IGS和COX1单倍型网络图及系统进化树则显示大浅滩种群的单倍型与其他地区单倍型之间亲缘关系较远。这些结果表明, 北大西洋东西两岸的二裂墨角藻可能在更新世末期经历了多次大规模灭绝, 北极的二裂墨角藻祖先可能在末次冰盛期之前的间冰期侵入到东北大西洋, 继而在随后的间冰期(如全新世)跨过大西洋侵入到北美。二裂墨角藻谱系多样性模式还显示纽芬兰大浅滩东岸的弗莱明角(Flemish Cap)可能是一个潜在的更新世末期冰期避难所。综上所述, 关键地区种群的谱系多样性结果可为深入理解海洋生物进化过程和模式提供重要线索, 进而为遗传资源评估、多样性保护和环境适应等提供科学指导。
张彤云, 胡自民 (2025) 二裂墨角藻谱系多样性模式显示纽芬兰大浅滩存在一个海洋冰期避难所. 生物多样性, 33, 24416. DOI: 10.17520/biods.2024416.
Tongyun Zhang, Zimin Hu (2025) The brown macroalga Fucus distichus revisited: Phylogeographic insights into a marine glacial refugium in the Grand Banks of Newfoundland, Canada. Biodiversity Science, 33, 24416. DOI: 10.17520/biods.2024416.
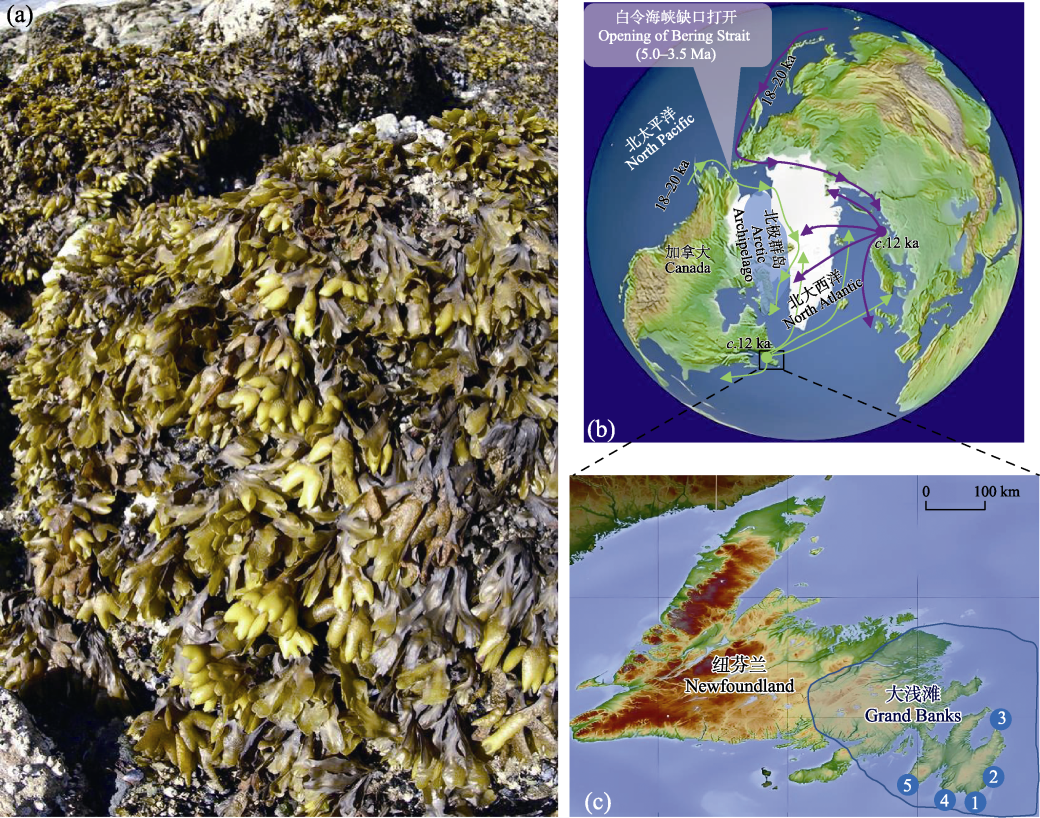
图1 二裂墨角藻简况。(a)二裂墨角藻外部形态及栖息地(加拿大不列颠哥伦比亚省温哥华)。(b)二裂墨角藻在泛北极地区的大致扩散历史。深紫色和绿色箭头显示二裂墨角藻北太平洋祖先在末次冰盛期两次跨北极侵入到北大西洋。挪威北部(绿色圆点)和加拿大纽芬兰(深紫色圆点)为北大西洋东西两岸的冰期避难所, 在此存留的祖先种群约在1.2万年前发生跨地区扩散形成现今分布格局。Ma: 百万年前; ka: 千年前。(c)纽芬兰大浅滩二裂墨角藻种群采样地点, 其中蓝线区域为大浅滩的大致范围。
Fig. 1 The brief description of the brown alga Fucus distichus. (a) The morphology and habitat of F. distichus (Vancouver, British Columbia, Canada); (b) The general dispersal history of F. distichus in the Pan-Arctic. The purple and green arrows indicate two separate trans-Arctic migration events of the ancestral F. distichus in the North Pacific into the North Atlantic. The purple and green circles represent two marine glacial refugia during the last glacial maximum on the northeast (northern Norway) and northwest (Newfoundland, Canada) Atlantic, where the survived ancestral populations expanded c. 12 ka to consequently form present-day distribution patterns. Ma, Million years ago; ka, Thousand years ago. (c) Sampling locations of F. distichus from the Grand Banks of Newfoundland, Canada.
| 采样地点 Sampling localities | 23S mtDNA基因间区 23S mtDNA intergenic spacer (IGS) | 细胞色素c氧化酶亚基 Cytochrome c oxidase subunit I (COX1) | 数据来源 Data source | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | h | π | hi | n | h | π | hi | ||
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB1) | 27 | 0.5014 | 0.0046 | Hap1 (i1)-Hap3 | 27 | 0.0000 | 0.0000 | Hap-1 (c1) | 本研究 This study |
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB2) | 6 | 0.7333 | 0.0054 | Hap1 (i1), Hap2, Hap5 | 10 | 0.0000 | 0.0000 | Hap-1 (c1) | 本研究 This study |
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB3) | 10 | 0.6889 | 0.0078 | Hap1 (i1)-Hap3 | 6 | 0.0000 | 0.0000 | Hap-1 (c1) | 本研究 This study |
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB5) | 29 | 0.6897 | 0.0088 | Hap1 (i1)-Hap4, Hap6-Hap10 | 24 | 0.3080 | 0.0036 | Hap-1 (c1) -Hap-4 | 本研究 This study |
| 加拿大纽芬兰拱门 Arches, Newfoundland, Canada | 27 | 0.0000 | 0.0000 | i1 | 12 | 0.3273 | 0.0008 | c1, c2 | Coyer et al, |
| 加拿大纽芬兰诺斯特德 Norstead, Newfoundland, Canada | 24 | 0.0000 | 0.0000 | i1 | 12 | 0.0000 | 0.0000 | c1 | Coyer et al, |
| 美国阿拉斯加阿图岛 Attu Island, Alaska, USA | 26 | 0.3624 | 0.0046 | i4, i5 | 12 | 0.0000 | 0.0000 | c1 | Coyer et al, |
| 美国阿拉斯加威廉王子湾 Prince Williams Sound, Alaska, USA | 26 | 0.5200 | 0.0138 | i4, i5, i7 | 12 | 0.1667 | 0.0004 | c1, c2 | Coyer et al, |
| 美国缅因阿普尔多尔岛 Appledore Island, Maine, USA | 24 | 0.0000 | 0.0000 | i1 | 12 | 0.5303 | 0.0013 | c1, c3 | Coyer et al, |
| 冰岛伊萨菲厄泽 Isafjörður, Iceland | 32 | 0.0000 | 0.0000 | i1 | 12 | 0.5303 | 0.0013 | c1, c2 | Coyer et al, |
| 冰岛格林达维克 Grindavik, Iceland | 28 | 0.3042 | 0.0017 | i1, i2 | 12 | 0.1667 | 0.0004 | c1, c2 | Coyer et al, |
| 冰岛布雷兹达斯维克 Breiðalsvik, Iceland | 30 | 0.0667 | 0.0004 | i1, i3 | 12 | 0.5000 | 0.0013 | c1, c2 | Coyer et al, |
| 丹麦法罗群岛斯特罗莫岛 Streymoy, Faroe Islands, Danmark | 16 | 0.2333 | 0.0013 | i1, i2 | 12 | 0.0000 | 0.0000 | c1 | Coyer et al, |
| 挪威哈默菲斯特 Hammerfest, Norway | 30 | 0.0000 | 0.0000 | i1 | 12 | 0.5303 | 0.0013 | c1, c2, c3 | Coyer et al, |
表1 纽芬兰大浅滩二裂墨角藻的遗传多样性(粗体字)与Coyer等(2011)研究中遗传多样性最高的种群(背景浅灰色)的比较
Table 1 Genetic diversity indices of the Fucus distichus populations sampled from Grand Banks of Newfoundland (values highlighted in bold) compared with the populations with the highest genetic diversity published by Coyer et al (2011) (background colored by light grey)
| 采样地点 Sampling localities | 23S mtDNA基因间区 23S mtDNA intergenic spacer (IGS) | 细胞色素c氧化酶亚基 Cytochrome c oxidase subunit I (COX1) | 数据来源 Data source | ||||||
|---|---|---|---|---|---|---|---|---|---|
| n | h | π | hi | n | h | π | hi | ||
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB1) | 27 | 0.5014 | 0.0046 | Hap1 (i1)-Hap3 | 27 | 0.0000 | 0.0000 | Hap-1 (c1) | 本研究 This study |
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB2) | 6 | 0.7333 | 0.0054 | Hap1 (i1), Hap2, Hap5 | 10 | 0.0000 | 0.0000 | Hap-1 (c1) | 本研究 This study |
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB3) | 10 | 0.6889 | 0.0078 | Hap1 (i1)-Hap3 | 6 | 0.0000 | 0.0000 | Hap-1 (c1) | 本研究 This study |
| 加拿大纽芬兰大浅滩 Grand banks, Newfoundland, Canada (GB5) | 29 | 0.6897 | 0.0088 | Hap1 (i1)-Hap4, Hap6-Hap10 | 24 | 0.3080 | 0.0036 | Hap-1 (c1) -Hap-4 | 本研究 This study |
| 加拿大纽芬兰拱门 Arches, Newfoundland, Canada | 27 | 0.0000 | 0.0000 | i1 | 12 | 0.3273 | 0.0008 | c1, c2 | Coyer et al, |
| 加拿大纽芬兰诺斯特德 Norstead, Newfoundland, Canada | 24 | 0.0000 | 0.0000 | i1 | 12 | 0.0000 | 0.0000 | c1 | Coyer et al, |
| 美国阿拉斯加阿图岛 Attu Island, Alaska, USA | 26 | 0.3624 | 0.0046 | i4, i5 | 12 | 0.0000 | 0.0000 | c1 | Coyer et al, |
| 美国阿拉斯加威廉王子湾 Prince Williams Sound, Alaska, USA | 26 | 0.5200 | 0.0138 | i4, i5, i7 | 12 | 0.1667 | 0.0004 | c1, c2 | Coyer et al, |
| 美国缅因阿普尔多尔岛 Appledore Island, Maine, USA | 24 | 0.0000 | 0.0000 | i1 | 12 | 0.5303 | 0.0013 | c1, c3 | Coyer et al, |
| 冰岛伊萨菲厄泽 Isafjörður, Iceland | 32 | 0.0000 | 0.0000 | i1 | 12 | 0.5303 | 0.0013 | c1, c2 | Coyer et al, |
| 冰岛格林达维克 Grindavik, Iceland | 28 | 0.3042 | 0.0017 | i1, i2 | 12 | 0.1667 | 0.0004 | c1, c2 | Coyer et al, |
| 冰岛布雷兹达斯维克 Breiðalsvik, Iceland | 30 | 0.0667 | 0.0004 | i1, i3 | 12 | 0.5000 | 0.0013 | c1, c2 | Coyer et al, |
| 丹麦法罗群岛斯特罗莫岛 Streymoy, Faroe Islands, Danmark | 16 | 0.2333 | 0.0013 | i1, i2 | 12 | 0.0000 | 0.0000 | c1 | Coyer et al, |
| 挪威哈默菲斯特 Hammerfest, Norway | 30 | 0.0000 | 0.0000 | i1 | 12 | 0.5303 | 0.0013 | c1, c2, c3 | Coyer et al, |
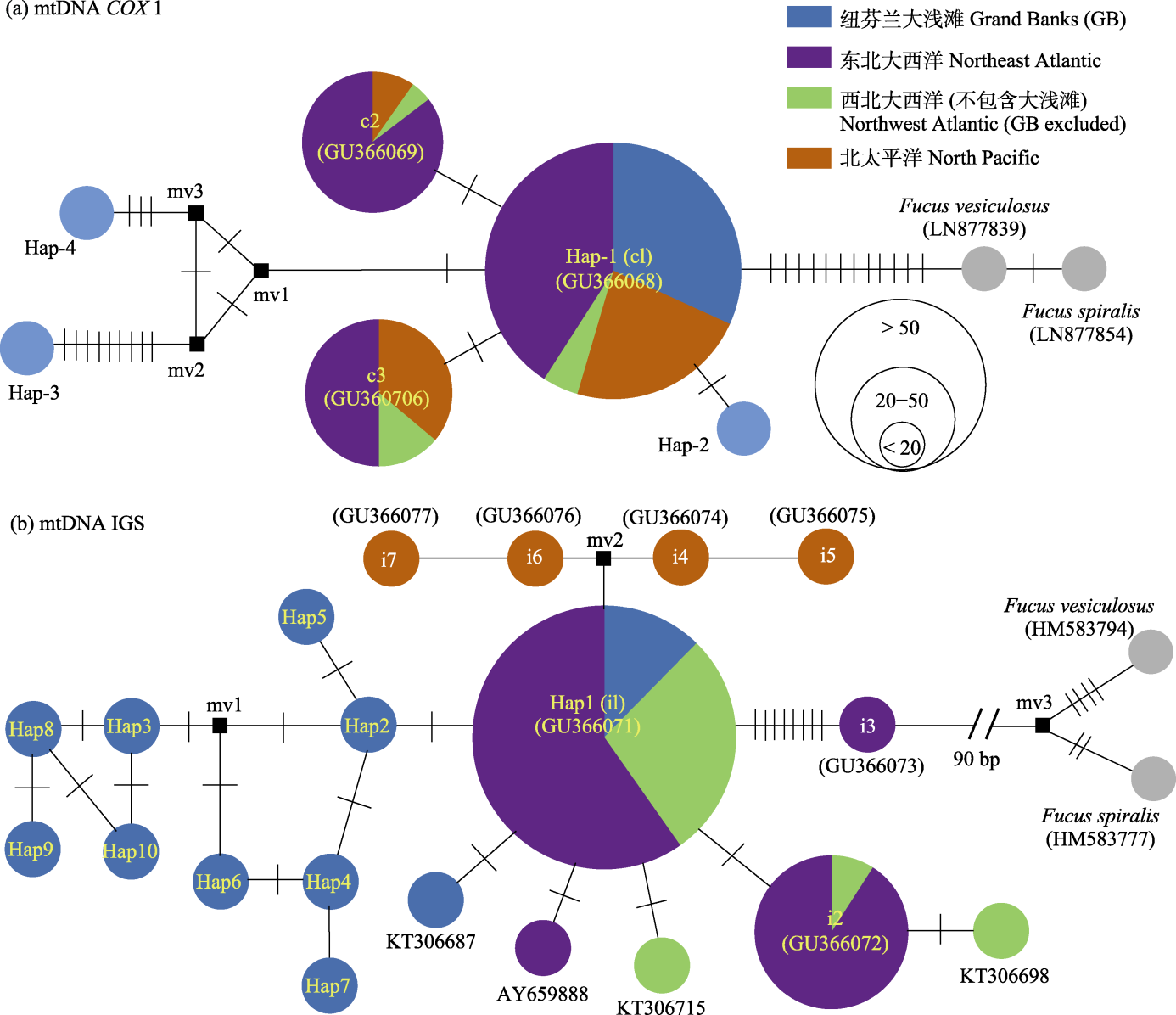
图2 基于mtDNA COX1 (a)和23S mtDNA IGS (b)构建的单倍型网络图。c1-c3和i1-i7分别对应Coyer等(2011)报道的COX1和IGS单倍型, 其中c1和i1分别与本研究中的Hap-1和Hap1相同。黑色方框(如mv)表示丧失或未采集到的单倍型。
Fig. 2 The constructed haplotype network based on mtDNA COX1 (a) and 23S mtDNA IGS datasets (b). c1-c3 and i1-i7 are COX1 and IGS haplotypes reported by Coyer et al (2011), respectively, of which c1 and i1 are identical to Hap-1 and Hap1, respectively identified in this study. The black boxes (e.g. mv) represent the lost or missed haplotypes.
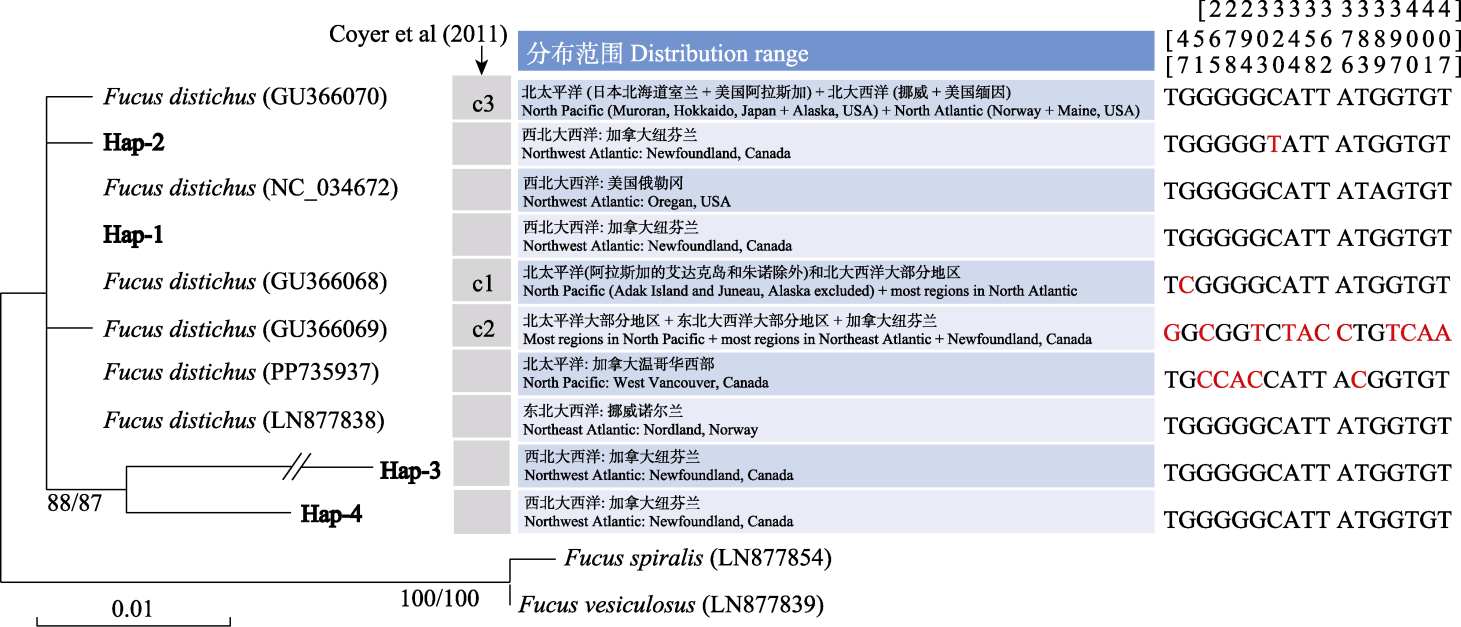
图3 基于mtDNA COX1单倍型构建的邻接系统进化树。字体加粗的Hap-1-Hap-4为本研究报道的纽芬兰大浅滩的单倍型。进化树上括号内的字符为相应序列的GenBank注册号, 斜线两侧的数字为邻接和最大似然算法自展值(1,000次重复)。箭头所示为Coyer等(2011)报道的COX1单倍型。每个COX1单倍型在不同位置(即比对碱基上方的数字)的碱基变异用红色凸显。
Fig. 3 Neighbour joining phylogenetic tree constructed using mtDNA COX1 haplotypes. Hap-1-Hap-4 in bold font are haplotypes identified from the Grand Banks of Newfoundland in this study. The characters in parentheses are GenBank accession numbers for each sequence, and the numbers on both sides of the slash are bootstrap values of neighbour joining and maximum likelihood algorithm (1,000 replicates). The arrow indicates the COX1 haplotypes reported by Coyer et al (2011). The nucleotide variations of each COX1 haplotype at different numbering sites (i.e. the numbers above the aligned nucleotides) are highlighted in red color.
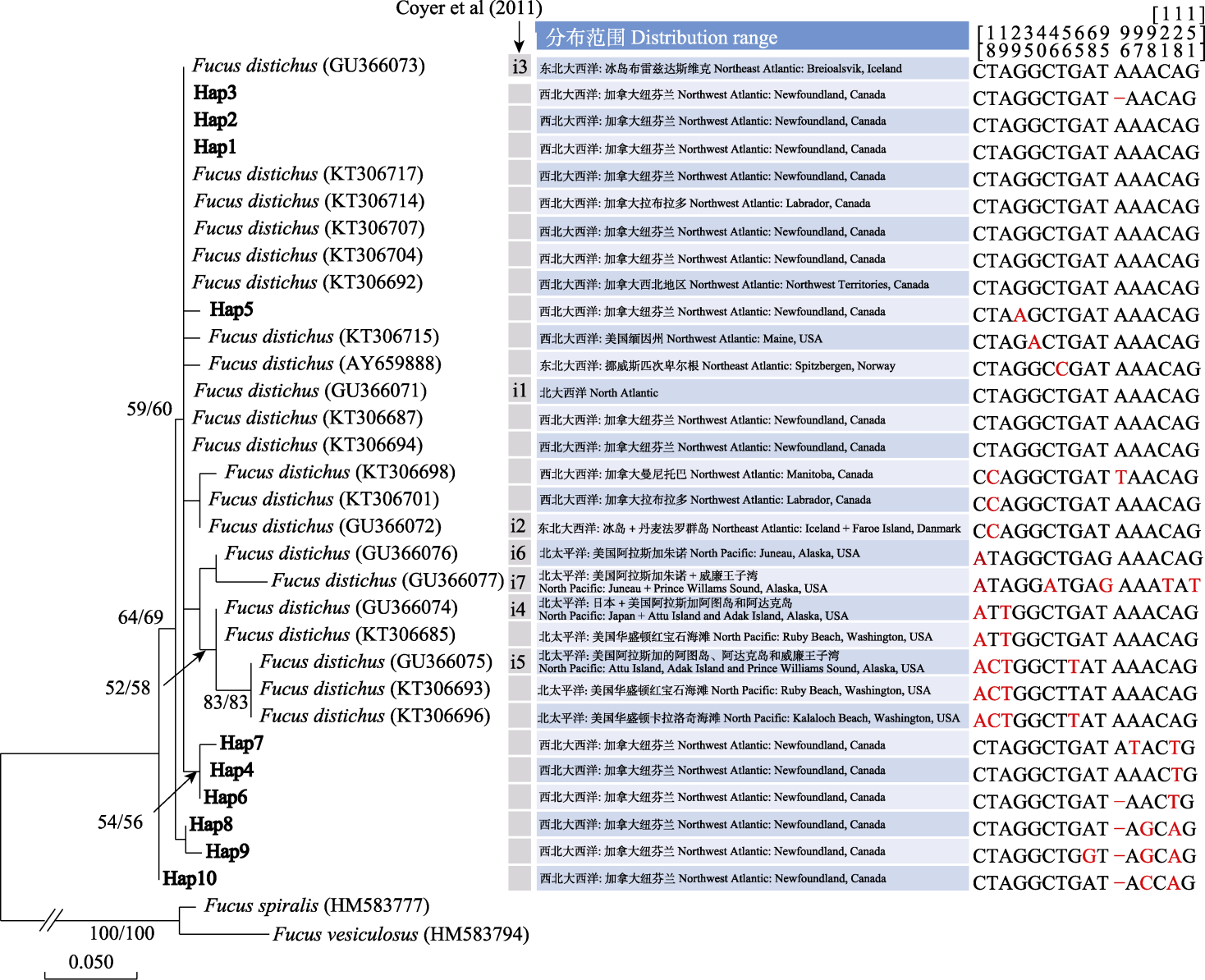
图4 基于23S mtDNA IGS单倍型构建的邻接系统进化树。加粗的Hap1-Hap10为本研究报道的纽芬兰大浅滩的单倍型。进化树上括号内的字符为相应序列的GenBank注册号, 斜线两侧的数字为邻接和最大似然算法自展值(1,000次重复)。箭头所示为Coyer等(2011)报道的IGS单倍型。核酸比对中的“-”表示碱基缺失。
Fig. 4 Neighbour joining phylogenetic tree constructed using 23S mtDNA IGS haplotypes. Hap1-Hap10 in bold font are haplotypes identified from the Grand Banks of Newfoundland in this study. The characters in parentheses are GenBank accession numbers for each sequence, and the numbers on both sides of the slash are bootstrap values of neighbour joining and maximum likelihood algorithm (1,000 replicates). The arrow indicates the IGS haplotypes reported by Coyer et al (2011). The nucleotide variations of each IGS haplotype at different numbering sites (i.e. the numbers above the aligned nucleotides) are highlighted in red color. “-” in aligned sequences indicates missed nucleotide.
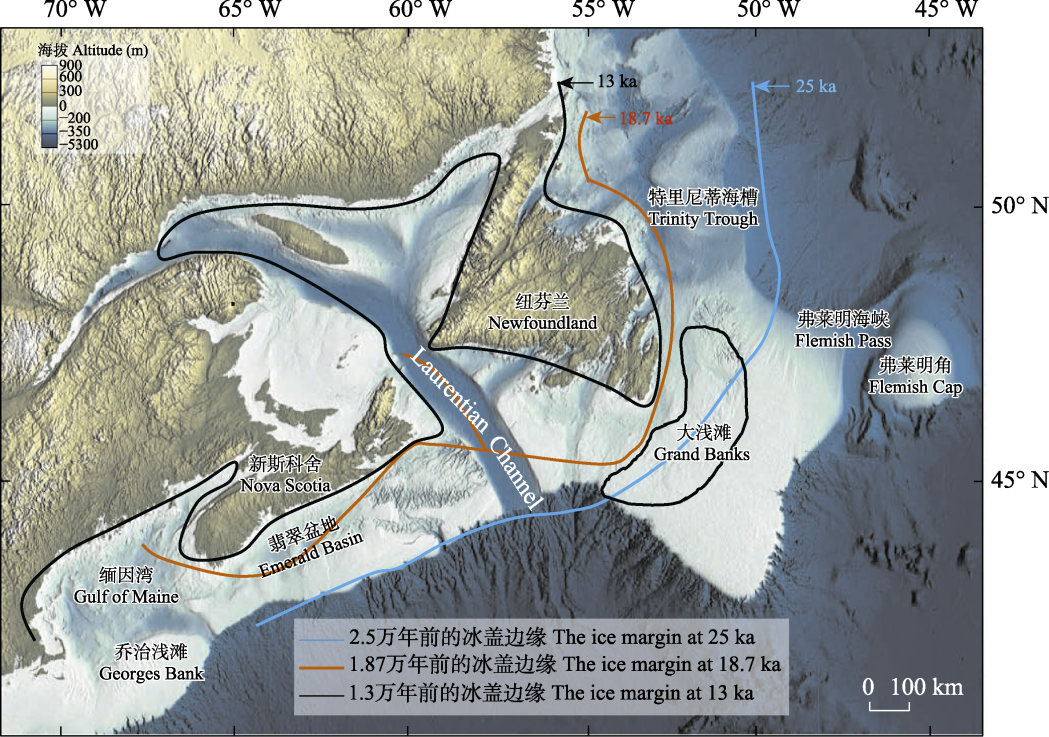
图5 弗莱明角相对于大浅滩的地理位置以及2.5万年前(蓝色线)、1.87万年前(褐色线)和1.3万年前(黑色线)纽芬兰周边冰盖边缘的大致位置。修改自Shaw (2006)和Fan等(2024)。ka: 千年前。
Fig. 5 The geographic location of the Flemish Cap relative to the Grand Banks, and the ice margin around Newfoundland at 25 ka (blue line), 18.7 ka (brown line), and 13 ka (black line). Modified from Shaw (2006) and Fan et al (2024). ka, Thousands years ago.
| [1] | Adey WH, Hayek LAC (2011) Elucidating marine biogeography with macrophytes: Quantitative analysis of the North Atlantic supports the thermogeographic model and demonstrates a distinct subarctic region in the northwestern Atlantic. Northeast Naturalist, 18, 1-125. |
| [2] | Adey WH, Lindstrom S, Hommersand M, Muller K (2008) The biogeographic origin of Arctic endemic sea weeds: A thermogeographic view. Journal of Phycology, 44, 1384-1394. |
| [3] | Allakhverdiev SI, Kreslavski VD, Klimov VV, Los DA, Carpentier R, Mohanty P (2008) Heat stress: An overview of molecular responses in photosynthesis. Photosynthetic Research, 98, 541-550. |
| [4] | Assis J, Araujo MB, Serrao EA (2018) Projected climate changes threaten ancient refugia of kelp forests in the North Atlantic. Global Change Biology, 24, e55-e66. |
| [5] |
Bandelt HJ, Forster P, Rohl A (1999) Median-joining networks for inferring intraspecific phylogenies. Molecular Biology and Evolution, 16, 37-48.
DOI PMID |
| [6] | Berndt ML, Callow JA, Brawley S (2002) Gamete concentrations and timing and success of fertilization in a rocky shore seaweed. Marine Ecology Progress Series, 226, 273-285. |
| [7] | Bird NL, McLachlan J (1976) Control of formation of receptacles in Fucus distichus L. subsp. distichus (Phaeophyceae, Fucales). Phycologia, 15, 79-84. |
| [8] | Brawley S, Coyer JA, Blakeslee AMH, Hoarau G, Johnson LE, Byers JE, Stam WT, Olsen JL (2009) Historical invasions of the intertidal zone of Atlantic North America associated with distinctive patterns of trade and emigration. Proceedings of the National Academy of Sciences, USA, 106, 8239-8244. |
| [9] | Briggs JC (2003) Marine centres of origin as evolutionary engines. Journal of Biogeography, 30, 1-18. |
| [10] |
Cánovas FG, Mota CF, Serrão EA, Pearson GA (2011) Driving south: A multi-gene phylogeny of the brown algal family Fucaceae reveals relationships and recent drivers of a marine radiation. BMC Evolutionary Biology, 11, 371.
DOI PMID |
| [11] | Catarino MD, Silva A, Cardoso SM (2018) Phycochemical constituents and biological activities of Fucus spp. Marine Drugs, 16, 249. |
| [12] | Coleman MA, Brawley SH (2005) Are life history characteristics good predictors of genetic diversity and structure? A case study of the intertidal alga Fucus spiralis (Heterokontophyta; Phaeophyceae). Journal of Phycology, 41, 753-762. |
| [13] | Coyer JA, Hoarau G, Oudot-Le Secq MP, Stam WT, Olsen JL (2006) A mtDNA-based phylogeny of the brown algal genus Fucus (Heterokontophyta; Phaeophyta). Molecular Phylogenetics and Evolution, 39, 209-222. |
| [14] | Coyer JA, Hoarau G, Schaik JV, Luijckx P, Olsen JL (2011) Trans-Pacific and trans-Arctic pathways of the intertidal macroalga Fucus distichus L. reveal multiple glacial refugia and colonizations from the North Pacific to the North Atlantic. Journal of Biogeography, 38, 756-771. |
| [15] |
Coyer JA, Hoarau G, Sjøtun K, Olsen JL (2008) Being abundant is not enough: A decrease in effective population size over eight generations in a Norwegian population of the seaweed, Fucus serratus. Biology Letters, 4, 755-757.
DOI PMID |
| [16] |
Coyer JA, Peters AF, Stam WT, Olsen JL (2003) Post-ice age recolonization and differentiation of Fucus serratus L. (Fucaceae: Phaeophyta) populations in Northern Europe. Molecular Ecology, 12, 1817-1829.
DOI PMID |
| [17] |
Cumashi A, Ushakova NA, Preobrazhenskaya ME, D’Incecco A, Piccoli A, Totani L, Tinari N, Morozevich GE, Berman AE, Bilan MI (2007) A comparative study of the anti-inflammatory, anticoagulant, antiangiogenic, and antiadhesive activities of nine different fucoidans from brown seaweeds. Glycobiology, 17, 541-552.
DOI PMID |
| [18] | Dyke AS, Prest VK (1987) Late Wisconsinan and Holocene history of the Laurentide Ice Sheet. Géographie Physique et Quaternaire, 41, 237-263. |
| [19] |
Engel CR, Daguin C, Serräo E (2005) Genetic entities and mating system in hermaphroditic Fucus spiralis and its close dioecious relative F. vesiculosus (Fucaceae, Phaeophyceae). Molecular Ecology, 14, 2033-2046.
DOI PMID |
| [20] |
Excoffier L, Lischer HEL (2010) Arlequin suite ver 3.5: A new series of programs to perform population genetics analyses under Linux and Windows. Molecular Ecology Resources, 10, 564-567.
DOI PMID |
| [21] | Fan RY, Gao Y, Xie XN, Gao YM, Su M (2024) Ichnological analysis of glacially-influenced sediments from the late Pleistocene to Holocene on the southeastern Canadian margin: Implications for palaeoclimate and palaeoceanography. Palaeogeography, Palaeoclimatology, Palaeoecology, 651, 112401. |
| [22] |
Fenberg PB, Posbic K, Hellberg ME (2014) Historical and recent processes shaping the geographic range of a rocky intertidal gastropod: Phylogeography, ecology, and habitat availability. Ecology and Evolution, 4, 3244-3255.
DOI PMID |
| [23] |
Ferreira JG, Arenas F, Marínez B, Hawkins SJ, Jenkins SR (2014) Physiological response of fucoid algae to environmental stress: Comparing range centre and southern populations. New Phytologist, 202, 1157-1172.
DOI PMID |
| [24] | Frenzel B, Pécsi M, Velichko AA (1992) Atlas of Paleoclimates and Paleoenvironments of the Northern Hemisphere: Late Pleistocene-Holocene. Geographical Research Institute, Hungarian Academy of Science, Budapest. |
| [25] | Hiscock K, Southward A, Tittley I, Hawkins S (2004) Effects of changing temperature on benthic marine life in Britain and Ireland. Aquatic Conservation, 14, 333-362. |
| [26] |
Hoarau G, Coyer JA, Veldsink JH, Stam WT, Olsen JL (2007) Glacial refugia and recolonization patterns in the brown seaweed Fucus serratus. Molecular Ecology, 16, 3606-3616.
DOI PMID |
| [27] | Hu ZM, Du YQ, Liang YS, Zhong KL, Zhang J (2021) Phylogeographic patterns and genetic connectivity of marine plants: A review. Oceanologia et Limnologia Sinica, 52, 418-432. (in Chinese with English abstract) |
| [胡自民, 杜玉群, 梁延硕, 钟凯乐, 张杰 (2021) 海洋植物谱系地理模式与遗传连通性研究进展. 海洋与湖沼, 52, 418-432.] | |
| [28] | Hu ZM, Guiry MD, Critchley AT, Duan DL (2010) Phylogeographic patterns indicate trans-Atlantic migration from Europe to North America in the red seaweed Chondrus crispus (Gigartinales, Rhodophyta). Journal of Phycology, 46, 889-900. |
| [29] | Hu ZM, Kantachumpoo A, Liu RY, Sun ZM, Yao JT, Komatusu T, Uwai S, Duan DL (2018) A late Pleistocene marine glacial refugium in the south-west of Hainan Island, China: Phylogeographical insights from the brown alga Sargassum polycystum. Journal of Biogeography, 45, 355-366. |
| [30] | Hu ZM, Uwai S, Yu SH, Komatsu T, Ajisaka T, Duan DL (2011) Phylogeographic heterogeneity of the brown macroalga Sargassum horneri (Fucaceae) in the northwestern Pacific in relation to late Pleistocene glaciation and tectonic configurations. Molecular Ecology, 20, 3894-3909. |
| [31] |
Jueterbock A, Smolina I, Coyer JA, Hoarau G (2016) The fate of the Arctic seaweed Fucus distichus under climate change: an ecological niche modelling approach. Ecology and Evolution, 6, 1712-1724.
DOI PMID |
| [32] | Kelly DW, Macisaac HJ, Heath DD, Crandall K (2009) Vicariance and dispersal effects on phylogeographic structure and speciation in a widespread estuarine invertebrate. Evolution, 60, 257-267. |
| [33] |
Kumar S, Stecher G, Li M, Knyaz C, Tamura K (2018) MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biology and Evolution, 35, 1547-1549.
DOI PMID |
| [34] | Laughinghouse HD IV, Müller KM, Adey WH, Lara Y, Young R, Johnson G (2015) Evolution of the northern rockweed, Fucus distichus, in a regime of glacial cycling: Implications for benthic algal phylogenetics. PLoS ONE, 10, e0143795. |
| [35] | Lindeberg MR, Lindstrom SC (2010) Field Guide to Seaweeds of Alaska. Fairbanks, Alaska. |
| [36] | Lindstrom SC (2001) The Bering Strait connection: Dispersal and speciation in boreal macroalgae. Journal of Biogeography, 28, 243-251. |
| [37] | Liu YJ, Zhong KL, Jueterbock A, Satoshi S, Choi HG, Weinberger F, Assis J, Hu ZM (2022) The invasive alga Gracilaria vermiculophylla in the native northwest Pacific under ocean warming: Southern genetic consequence and northern range expansion. Frontiers in Marine Science, 9, 983685. |
| [38] | Maggs CA, Castilho R, Foltz DW, Henzler C, Jolly MT, Kelly J, Olsen JL, Perez KE, Stam WT, Vainola R, Viard F, Wares JP (2008) Evaluating signatures of glacial refugia for north Atlantic benthic marine taxa. Ecology, 89, S108-S122. |
| [39] | Marincovich L, Gladenov A (1999) Evidence for an earlier opening of the Bering Strait. Nature, 397, 149-151. |
| [40] | McCabe MK, Konar B (2021) Influence of environmental attributes on intertidal community structure in glacial estuaries. Deep Sea Research Part II: Topical Studies in Oceanography, 194, 104986. |
| [41] | Norton TA (1992) Dispersal by macroalgae. British Phycological Journal, 27, 293-301. |
| [42] | Olsen JL, Zechman FW, Hoarau G, Coyer JA, Stam WT, Valero M, Åberg P (2010) The phylogeographic architecture of the fucoid seaweed Ascophyllum nodosum: An intertidal ‘marine tree’ and survivor of more than one glacial- interglacial cycle. Journal of Biogeography, 37, 842-856. |
| [43] | Pearson G, Brawley S (1996) Reproductive ecology of Fucus distichus (Phaeophyceae): An intertidal alga with successful external fertilization. Marine Ecology Progress Series, 143, 211-223. |
| [44] |
Pereyra RT, Bergström L, Kautsky L, Johannesson K (2009) Rapid speciation in a newly opened postglacial marine environment, the Baltic Sea. BMC Evolutionary Biology, 9, 70.
DOI PMID |
| [45] | Popescu SM, Suc JP, Fauquette S, Bessedik M, Jimenez- Moreno G, Robin C, Labrousse L (2021) Mangrove distribution and diversity during three Cenozoic thermal maxima in the Northern Hemisphere (pollen records from the Arctic-North Atlantic-Mediterranean regions). Journal of Biogeography, 48, 2771-2784. |
| [46] | Rozas J, Ferrer-Mata A, Sánchez-DelBarrio JC, Guirao-Rico S, Librado P, Ramos-Onsins SE, Sánchez-Gracia A (2017) DnaSP 6: DNA sequence polymorphism analysis of large datasets. Molecular Biology and Evolution, 34, 3299-3302. |
| [47] | Serräo E, Alice L, Brawley S (1999) Evolution of the Fucaceae (Phaeophyceae) inferred from nrDNA-ITS. Journal of Phycology, 35, 382-394. |
| [48] | Serräo EA, Kautsky L, Lifvergren T, Brawley S (1997) Gamete dispersal and pre-recruitment mortality in Baltic Fucus vesiculosus. Phycologia (Suppl.), 36, 101-102. |
| [49] | Shaw J (2003) Submarine moraines in Newfoundland coastal waters: Implications for the deglaciation of Newfoundland and adjacent areas. Quaternary International, 99/100, 115-134. |
| [50] | Shaw J (2006) Palaeogeography of Atlantic Canadian continental shelves from the last glacial maximum to the present, with an emphasis on Flemish Cap. Journal of Northwest Atlantic Fishery Science, 37, 119-126. |
| [51] | Shaw J, Piper DJW, Fader GBJ, King EL, Todd BJ, Bell T, Batterson MJ, Liverman DGE (2006) A conceptual model of the deglaciation of Atlantic Canada. Quaternary Science Reviews, 25, 2059-2081. |
| [52] | Signore AV, Morrison PR, Brauner CJ, Fago A, Weber RE, Campbell KL (2023) Evolution of an extreme hemoglobin phenotype contributed to the sub-Arctic specialization of extinct Steller’s sea cows. eLife, 12, e85414. |
| [53] | Smolina I, Kollias S, Jueterbock A, Coyer JA, Hoarau G (2016) Variation in thermal stress response in two populations of the brown seaweed, Fucus distichus, from the Arctic and subarctic intertidal. Royal Society Open Science, 3, 150429. |
| [54] | Song XH, Assis J, Zhang J, Gao X, Choi HG, Duan DL, Serrao EA, Hu ZM (2021) Climate-induced range shifts shaped the present and threaten the future genetic variability of a marine brown alga in the Northwest Pacific. Evolutionary Applications, 14, 1867-1879. |
| [55] | Song XK, Gravili C, Wang JJ, Deng YC, Wang YQ, Fang L, Lin HS, Wang SQ, Zheng YT, Lin JH (2016) A new deep-sea hydroid (Cnidaria: Hydrozoa) from the Bering Sea Basin reveals high genetic relevance to Arctic and adjacent shallow-water species. Polar Biology, 39, 461-471. |
| [56] | Sukhoveeva MV, Podkorytova AV (2006) Commercial Algae and Grasses of the Seas of the Far East: Biology, Distribution, Stocks, Processing Technology. Tinro-Center, Vladivostok. |
| [57] | Svendsen H, Beszczynska-Møller A, Hagen JO, Lefauconnier B, Tverberg V, Gerland S (2002) The physical environment of Kongsfjorden-Krossfjorden, an Arctic fjord system in Svalbard. Polar Research, 21, 133-166. |
| [58] |
Tatarenkov A, Bergström L, Jönsson RB, Serrao EA, Kautsky L, Johannesson K (2005) Intriguing asexual life in marginal populations of the brown seaweed Fucus vesiculosus. Molecular Ecology, 14, 647-651.
DOI PMID |
| [59] | Umanzor S, Sandoval-Gil JM, Conitz J (2023) Ecophysiological responses of the intertidal seaweed Fucus distichus to temperature changes and reduced light driven by tides and glacial input. Estuaries and Coasts, 46, 1269-1279. |
| [60] | van Oppen MJH, Draisma SGA, Olsen JL, Stam WT (1995) Multiple trans-Arctic passages in the red alga Phycodrys rubens: Evidence from nuclear rDNA ITS sequences. Marine Biology, 123, 179-188. |
| [61] | Vermeij GJ (2005) From Europe to America:Pliocene to recent trans-Atlantic expansion of cold-water North Atlantic molluscs. Proceedings of the Royal Society B: Biological Sciences, 272, 2545-2550. |
| [62] | Wahl M, Jormalaineny V, Erikssonz BK, Coyer JA, Molis M, Schubert H, Dethier M, Karez R, Kruse I, Lenz M, Pearson G, Rohde S, Wikström SA, Olsen JL (2011) Stress ecology in Fucus:Abiotic, biotic and genetic interactions. In: Advances in Marine Biology (ed. Lesser M), pp. 37-105. Academic Press, Oxford. |
| [63] |
Wares JP, Cunningham CW (2001) Phylogeography and historical ecology of the North Atlantic intertidal. Evolution, 55, 2455-2469.
DOI PMID |
| [64] | Weslawski JM, Wiktor J, Kotwicki L (2010) Increase in biodiversity in the Arctic rocky littoral, Sorkappland, Svalbard, after 20 years of climate warming. Marine Biodiversity, 40, 123-130. |
| [65] | Zhong KL, Song XH, Choi HG, Satoshi S, Weinberger F, Draisma SGA, Duan DL, Hu ZM (2020) MtDNA-based phylogeography of the red alga Agarophyton vermiculophyllum (Gigartinales, Rhodophyta) in the native Northwest Pacific. Frontiers in Marine Science, 7, 366. |
| [1] | 徐进博, 崔雅倩, 王渊, 王伟波, 刘锋, 王广龙, 扈晶晶, 普布顿珠, 边巴多吉, 旦增, 胡开, 王小川, 宋刚, 吕永磊, 温知新. 西藏雅鲁藏布大峡谷国家级自然保护区内白颊猕猴的栖息地适宜性评价[J]. 生物多样性, 2025, 33(7): 24493-. |
| [2] | 顾婧婧, 刘宜卓, 苏杨. 基层地方政府在完成《昆蒙框架》中的作用和难点: 基于《联合国气候变化框架公约》任务的比较[J]. 生物多样性, 2025, 33(3): 24585-. |
| [3] | 吴琪, 张晓青, 杨雨婷, 周艺博, 马毅, 许大明, 斯幸峰, 王健. 浙江钱江源-百山祖国家公园庆元片区叶附生苔多样性及其时空变化[J]. 生物多样性, 2024, 32(4): 24010-. |
| [4] | 曹可欣, 王敬雯, 郑国, 武鹏峰, 李英滨, 崔淑艳. 降水格局改变及氮沉降对北方典型草原土壤线虫多样性的影响[J]. 生物多样性, 2024, 32(3): 23491-. |
| [5] | 龙诗怡, 张博博, 夏宇辰, 费杨帆, 孟亚妮, 吕冰薇, 宋月青, 郑普, 郭陶然, 张健, 黎绍鹏. 本地群落多样性和时间稳定性对加拿大一枝黄花生物量的影响[J]. 生物多样性, 2024, 32(11): 24263-. |
| [6] | 冯莉. 国际法视野下生物多样性和气候变化的协同治理[J]. 生物多样性, 2023, 31(7): 23110-. |
| [7] | 肖媛媛, 冯薇, 乔艳桂, 张宇清, 秦树高. 固沙灌木林地土壤微生物群落特征对土壤多功能性的影响[J]. 生物多样性, 2023, 31(4): 22585-. |
| [8] | 姚雪, 陈星, 戴尊, 宋坤, 邢诗晨, 曹宏彧, 邹璐, 王健. 采集策略对叶附生苔类植物发现概率及物种多样性的重要性[J]. 生物多样性, 2023, 31(4): 22685-. |
| [9] | 邵雯雯, 范国祯, 何知舟, 宋志平. 多地同质园实验揭示普通野生稻的表型可塑性与本地适应性[J]. 生物多样性, 2023, 31(3): 22311-. |
| [10] | 桑佳文, 宋创业, 贾宁霞, 贾元, 刘长成, 乔鲜果, 张琳, 袁伟影, 吴冬秀, 李凌浩, 郭柯. 青藏高原植被调查与制图评估[J]. 生物多样性, 2023, 31(3): 22430-. |
| [11] | 王金洲, 徐靖. “基于自然的解决方案”应对生物多样性丧失和气候变化: 进展、挑战和建议[J]. 生物多样性, 2023, 31(2): 22496-. |
| [12] | 李季蔓, 靳楠, 胥毛刚, 霍举颂, 陈小云, 胡锋, 刘满强. 不同干旱水平下蚯蚓对番茄抗旱能力的影响[J]. 生物多样性, 2022, 30(7): 21488-. |
| [13] | 朱瑞良, 马晓英, 曹畅, 曹子寅. 中国苔藓植物多样性研究进展[J]. 生物多样性, 2022, 30(7): 22378-. |
| [14] | 祖奎玲, 王志恒. 山地物种海拔分布对气候变化响应的研究进展[J]. 生物多样性, 2022, 30(5): 21451-. |
| [15] | 井新, 蒋胜竞, 刘慧颖, 李昱, 贺金生. 气候变化与生物多样性之间的复杂关系和反馈机制[J]. 生物多样性, 2022, 30(10): 22462-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
![]()

