



生物多样性 ›› 2021, Vol. 29 ›› Issue (10): 1348-1357. DOI: 10.17520/biods.2021121 cstr: 32101.14.biods.2021121
收稿日期:2021-04-01
接受日期:2021-05-11
出版日期:2021-10-20
发布日期:2021-10-20
通讯作者:
孙锋
作者简介:* E-mail: sf0204@163.com基金资助:Received:2021-04-01
Accepted:2021-05-11
Online:2021-10-20
Published:2021-10-20
Contact:
Feng Sun
摘要:
随着全球气候变暖, 我国岷江上游干旱区面积呈现增加的趋势。花椒(Zanthoxylum bungeanum)是岷江上游重要的经济树种之一, 对当地经济和社会发展起着重要作用, 提高花椒生态系统应对干旱干扰已成为迫切的问题。本研究设置了花椒单作、花椒-苜蓿(Medicago sativa)间作和花椒-大豆(Glycine max)间作3种种植模式, 在2015年8月对每种种植模式模拟干旱30 d, 每种种植模式包括干旱和对照处理, 在模拟干旱结束后、恢复15 d、30 d和45 d后分别采集土壤样品, 分析土壤化学性质、土壤微生物和线虫群落, 以探究花椒林下豆科植物能否缓和干旱的遗留效应对土壤化学性质和土壤生物的影响。重复测量方差分析表明: 在花椒单作模式下, 干旱恢复45 d后土壤硝态氮含量显著高于对照, 微生物量和真菌/细菌比与对照无显著差异, 线虫密度与对照无显著差异, 但线虫功能团没有恢复到对照水平; 在花椒-苜蓿间作模式下, 干旱恢复45 d后土壤含水量、铵态氮、硝态氮、溶解性有机碳、溶解性有机氮、微生物量、真菌/细菌比、线虫密度和线虫功能团组成与对照无显著差异, 但植食性线虫属Boleodorus相对多度显著高于对照; 在花椒-大豆间作模式下, 干旱恢复45 d后土壤含水量、铵态氮、硝态氮、溶解性有机碳、溶解性有机氮、微生物量和真菌/细菌比与对照无显著差异, 但线虫密度和功能团组成与对照有显著差异。在3种花椒种植模式中, 花椒-苜蓿间作模式下干旱的遗留效应对土壤养分和生物的影响最小。因此, 在干旱背景下, 花椒林下间作豆科植物可以加快土壤养分、土壤微生物和线虫群落的恢复, 进而有利于目标作物生长。
宋成军, 孙锋 (2021) 干旱对不同花椒种植模式下土壤微生物和线虫群落的影响. 生物多样性, 29, 1348-1357. DOI: 10.17520/biods.2021121.
Chengjun Song, Feng Sun (2021) Effects of Zanthoxylum bungeanum agroforestry systems on soil microbial and nematode communities under drought. Biodiversity Science, 29, 1348-1357. DOI: 10.17520/biods.2021121.
| 恢复时间 Recovery days | 种植模式 Planting system | 处理 Treatment | 土壤含水量 SWC (%) | 铵态氮 NH4+-N (mg/kg) | 硝态氮 NO3--N (mg/kg) | 溶解性有机碳 DOC (mg/kg) | 溶解性有机氮 DON (mg/kg) |
|---|---|---|---|---|---|---|---|
| 0天 | Z | 对照 Control | 18.63 ± 0.80 | 4.16 ± 0.41 | 17.81 ± 0.45 | 212.88 ± 1.06 | 55.25 ± 0.49 |
| 0 day | 干旱 Drought | 12.47 ± 0.48 | 3.53 ± 0.84 | 25.16 ± 1.24 | 213.38 ± 4.65 | 59.30 ± 3.84 | |
| Z-M | 对照 Control | 19.42 ± 0.67 | 6.07 ± 0.86 | 24.94 ± 0.71 | 237.94 ± 7.07 | 64.89 ± 2.25 | |
| 干旱 Drought | 13.33 ± 0.64 | 4.29 ± 0.99 | 27.40 ± 3.07 | 229.45 ± 4.16 | 65.06 ± 2.17 | ||
| Z-G | 对照 Control | 19.14 ± 0.54 | 4.31 ± 0.49 | 25.19 ± 1.12 | 229.37 ± 1.98 | 62.83 ± 0.89 | |
| 干旱 Drought | 12.76 ± 0.55 | 3.98 ± 0.66 | 17.14 ± 1.82 | 198.59 ± 3.09 | 55.25 ± 1.68 | ||
| 15天 | Z | 对照 Control | 18.10 ± 0.53 | 3.06 ± 0.15 | 21.22 ± 0.70 | 195.66 ± 9.98 | 49.88 ± 0.43 |
| 15 days | 干旱 Drought | 18.40 ± 0.59 | 3.18 ± 0.08 | 30.92 ± 1.84 | 191.35 ± 4.19 | 50.34 ± 2.14 | |
| Z-M | 对照 Control | 19.74 ± 1.21 | 4.16 ± 0.28 | 26.57 ± 2.40 | 263.98 ± 42.91 | 61.58 ± 4.50 | |
| 干旱 Drought | 19.63 ± 0.64 | 3.33 ± 0.14 | 27.80 ± 2.05 | 237.18 ± 10.26 | 60.00 ± 2.31 | ||
| Z-G | 对照 Control | 18.93 ± 0.94 | 3.43 ± 0.19 | 28.56 ± 2.01 | 196.09 ± 13.38 | 55.99 ± 1.18 | |
| 干旱 Drought | 19.00 ± 0.89 | 3.10 ± 0.12 | 22.61 ± 0.66 | 165.92 ± 3.99 | 57.34 ± 1.38 | ||
| 30天 | Z | 对照 Control | 18.03 ± 0.57 | 3.65 ± 0.18 | 18.72 ± 1.15 | 174.54 ± 15.17 | 51.79 ± 1.04 |
| 30 days | 干旱 Drought | 17.13 ± 0.52 | 3.15 ± 0.10 | 30.93 ± 1.52 | 170.91 ± 8.35 | 50.50 ± 1.89 | |
| Z-M | 对照 Control | 19.34 ± 0.96 | 4.65 ± 0.22 | 27.97 ± 1.62 | 188.71 ± 11.75 | 58.00 ± 3.69 | |
| 干旱 Drought | 18.13 ± 0.58 | 3.29 ± 0.15 | 27.45 ± 2.01 | 189.41 ± 8.67 | 55.87 ± 1.14 | ||
| Z-G | 对照 Control | 19.24 ± 0.76 | 3.99 ± 0.22 | 25.76 ± 1.19 | 173.52 ± 4.84 | 52.48 ± 4.58 | |
| 干旱 Drought | 18.30 ± 0.95 | 3.09 ± 0.17 | 23.90 ± 1.31 | 171.47 ± 9.69 | 54.09 ± 3.18 | ||
| 45天 | Z | 对照 Control | 17.91 ± 0.36 | 2.31 ± 0.62 | 18.57 ± 2.24 | 152.36 ± 2.17 | 48.04 ± 2.49 |
| 45 days | 干旱 Drought | 17.27 ± 0.32 | 1.88 ± 0.39 | 26.03 ± 2.58 | 154.61 ± 3.95 | 48.42 ± 2.48 | |
| Z-M | 对照 Control | 19.45 ± 0.80 | 1.98 ± 0.66 | 23.25 ± 1.27 | 179.35 ± 5.66 | 52.89 ± 3.81 | |
| 干旱 Drought | 19.37 ± 1.06 | 1.94 ± 0.54 | 27.67 ± 2.46 | 181.62 ± 12.04 | 54.93 ± 3.07 | ||
| Z-G | 对照 Control | 19.52 ± 1.19 | 2.27 ± 0.64 | 21.98 ± 2.54 | 165.24 ± 7.26 | 53.20 ± 1.75 | |
| 干旱 Drought | 18.30 ± 0.59 | 2.09 ± 0.44 | 23.25 ± 1.92 | 167.78 ± 9.66 | 53.12 ± 3.36 | ||
| 双因素重复测量方差分析 Two-way repeated ANOVA | |||||||
| Z | ns | ns | ns | P = 0.007 | ns | ns | |
| Z-M | ns | ns | ns | ns | ns | ns | |
| Z-G | ns | ns | ns | ns | ns | ns | |
表1 花椒单作、花椒-苜蓿间作和花椒-大豆间作种植模式下各取样期土壤化学性质
Table 1 Soil chemical properties in monocultures of the focal species Zanthoxylum bungeanum, mixed cultures of Z. bungeanum and Medicago sativa, and mixed cultures of Z. bungeanum and Glycine max at each sampling time.
| 恢复时间 Recovery days | 种植模式 Planting system | 处理 Treatment | 土壤含水量 SWC (%) | 铵态氮 NH4+-N (mg/kg) | 硝态氮 NO3--N (mg/kg) | 溶解性有机碳 DOC (mg/kg) | 溶解性有机氮 DON (mg/kg) |
|---|---|---|---|---|---|---|---|
| 0天 | Z | 对照 Control | 18.63 ± 0.80 | 4.16 ± 0.41 | 17.81 ± 0.45 | 212.88 ± 1.06 | 55.25 ± 0.49 |
| 0 day | 干旱 Drought | 12.47 ± 0.48 | 3.53 ± 0.84 | 25.16 ± 1.24 | 213.38 ± 4.65 | 59.30 ± 3.84 | |
| Z-M | 对照 Control | 19.42 ± 0.67 | 6.07 ± 0.86 | 24.94 ± 0.71 | 237.94 ± 7.07 | 64.89 ± 2.25 | |
| 干旱 Drought | 13.33 ± 0.64 | 4.29 ± 0.99 | 27.40 ± 3.07 | 229.45 ± 4.16 | 65.06 ± 2.17 | ||
| Z-G | 对照 Control | 19.14 ± 0.54 | 4.31 ± 0.49 | 25.19 ± 1.12 | 229.37 ± 1.98 | 62.83 ± 0.89 | |
| 干旱 Drought | 12.76 ± 0.55 | 3.98 ± 0.66 | 17.14 ± 1.82 | 198.59 ± 3.09 | 55.25 ± 1.68 | ||
| 15天 | Z | 对照 Control | 18.10 ± 0.53 | 3.06 ± 0.15 | 21.22 ± 0.70 | 195.66 ± 9.98 | 49.88 ± 0.43 |
| 15 days | 干旱 Drought | 18.40 ± 0.59 | 3.18 ± 0.08 | 30.92 ± 1.84 | 191.35 ± 4.19 | 50.34 ± 2.14 | |
| Z-M | 对照 Control | 19.74 ± 1.21 | 4.16 ± 0.28 | 26.57 ± 2.40 | 263.98 ± 42.91 | 61.58 ± 4.50 | |
| 干旱 Drought | 19.63 ± 0.64 | 3.33 ± 0.14 | 27.80 ± 2.05 | 237.18 ± 10.26 | 60.00 ± 2.31 | ||
| Z-G | 对照 Control | 18.93 ± 0.94 | 3.43 ± 0.19 | 28.56 ± 2.01 | 196.09 ± 13.38 | 55.99 ± 1.18 | |
| 干旱 Drought | 19.00 ± 0.89 | 3.10 ± 0.12 | 22.61 ± 0.66 | 165.92 ± 3.99 | 57.34 ± 1.38 | ||
| 30天 | Z | 对照 Control | 18.03 ± 0.57 | 3.65 ± 0.18 | 18.72 ± 1.15 | 174.54 ± 15.17 | 51.79 ± 1.04 |
| 30 days | 干旱 Drought | 17.13 ± 0.52 | 3.15 ± 0.10 | 30.93 ± 1.52 | 170.91 ± 8.35 | 50.50 ± 1.89 | |
| Z-M | 对照 Control | 19.34 ± 0.96 | 4.65 ± 0.22 | 27.97 ± 1.62 | 188.71 ± 11.75 | 58.00 ± 3.69 | |
| 干旱 Drought | 18.13 ± 0.58 | 3.29 ± 0.15 | 27.45 ± 2.01 | 189.41 ± 8.67 | 55.87 ± 1.14 | ||
| Z-G | 对照 Control | 19.24 ± 0.76 | 3.99 ± 0.22 | 25.76 ± 1.19 | 173.52 ± 4.84 | 52.48 ± 4.58 | |
| 干旱 Drought | 18.30 ± 0.95 | 3.09 ± 0.17 | 23.90 ± 1.31 | 171.47 ± 9.69 | 54.09 ± 3.18 | ||
| 45天 | Z | 对照 Control | 17.91 ± 0.36 | 2.31 ± 0.62 | 18.57 ± 2.24 | 152.36 ± 2.17 | 48.04 ± 2.49 |
| 45 days | 干旱 Drought | 17.27 ± 0.32 | 1.88 ± 0.39 | 26.03 ± 2.58 | 154.61 ± 3.95 | 48.42 ± 2.48 | |
| Z-M | 对照 Control | 19.45 ± 0.80 | 1.98 ± 0.66 | 23.25 ± 1.27 | 179.35 ± 5.66 | 52.89 ± 3.81 | |
| 干旱 Drought | 19.37 ± 1.06 | 1.94 ± 0.54 | 27.67 ± 2.46 | 181.62 ± 12.04 | 54.93 ± 3.07 | ||
| Z-G | 对照 Control | 19.52 ± 1.19 | 2.27 ± 0.64 | 21.98 ± 2.54 | 165.24 ± 7.26 | 53.20 ± 1.75 | |
| 干旱 Drought | 18.30 ± 0.59 | 2.09 ± 0.44 | 23.25 ± 1.92 | 167.78 ± 9.66 | 53.12 ± 3.36 | ||
| 双因素重复测量方差分析 Two-way repeated ANOVA | |||||||
| Z | ns | ns | ns | P = 0.007 | ns | ns | |
| Z-M | ns | ns | ns | ns | ns | ns | |
| Z-G | ns | ns | ns | ns | ns | ns | |
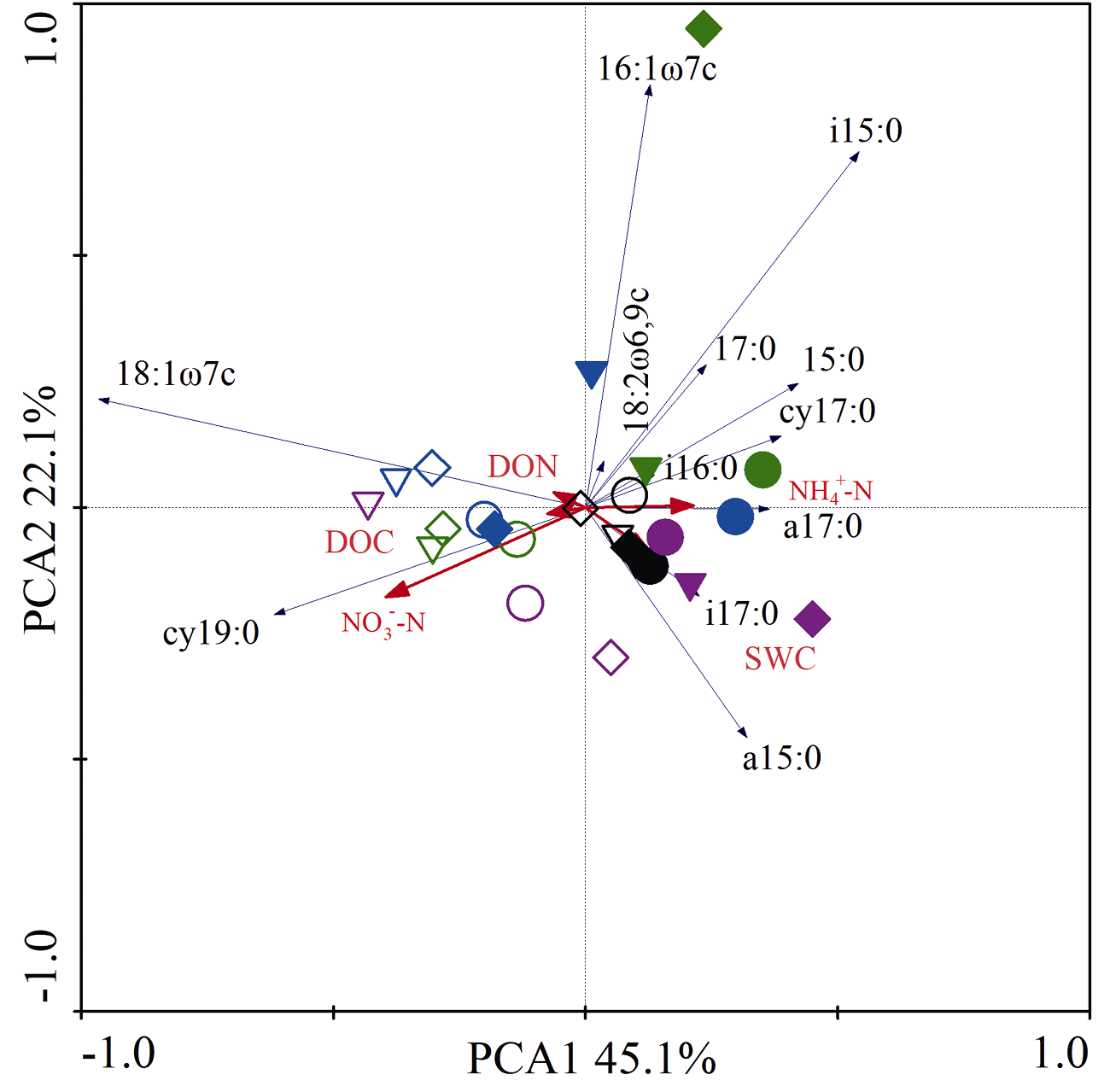
图2 微生物群落的主成分分析。SWC: 土壤含水量; DOC: 溶解性有机碳; DON: 溶解性有机氮。圆形为花椒单作, 菱形为花椒-苜蓿间作, 三角形为花椒-大豆间作。黑色、绿色、紫色和蓝色图形符号分别表示干旱后恢复0、15、30和45天。空心表示对照, 实心表示干旱。
Fig. 2 Principal component analysis (PCA) of the microbial community. SWC is soil water content; DOC and DON are the dissolved organic carbon and nitrogen, respectively. Circle indicates the monoculture of Zanthoxylum bungeanum, diamond indicates the mixed cultures of Z. bungeanum and Medicago sativa, triangle indicates the mixed cultures of Z. bungeanum and Glycine max. Black, green, purple and blue symbols represent 0, 15, 30 and 45 days of recovery after drought, respectively. Hollow indicates control, solid indicates drought.
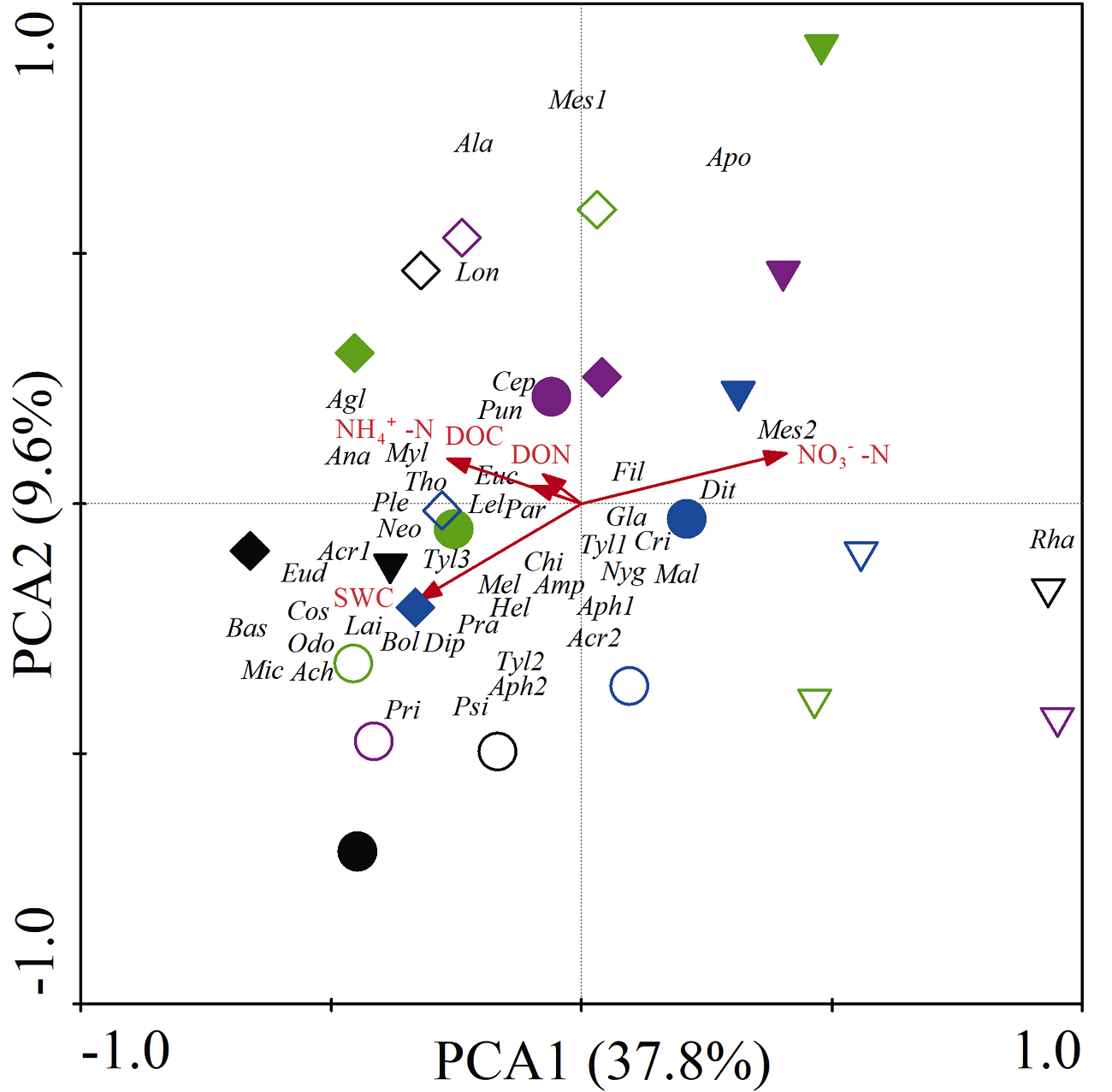
图3 线虫群落主成分分析。SWC: 土壤含水量; DOC: 溶解性有机碳; DON: 溶解性有机氮。图形符号含义见图2。黑色、绿色、紫色和蓝色图形符号分别表示干旱后恢复0、15、30和45天。空心表示对照, 实心表示干旱。Tyl1: 垫刃属; Mal: 剑尾垫刃属; Psi: 平滑垫刃属; Agl: 野外垫刃属; Fil: 丝尾垫刃属; Cos: 具脊垫刃属; Lel: 细纹垫刃属; Bol: 叉针属; Par: 针属; Neo: 新萨达属; Pra: 短体属; Tyl2: 矮化属; Cri: 中轮属; Mel: 根结属; Hel: 螺旋属; Lon: 长针属; Xip: 剑属; Rha: 小杆属; Mes2: 中杆属; Odo: 齿腔属; Gla: Glauxinema; Acr1: 丽突属; Acr2: 拟丽突属; Cep: 头叶属; Euc: 真头叶属; Ple: 绕线属; Ana: 拟绕线属; Chi: 板唇属; Cer: 鹿角唇属; Chr: 连胃属; Bas: 巴氏属; Pri: 棱咽属; Ala: 无咽属; Amp: 高杯侧属; Aph1: 滑刃属; Aph2: 真滑刃属; Dit: 茎属; Dip: 膜皮属; Tyl3: 垫咽属; Tri: 三孔属; Myl: 锉齿属; Ach: 异色矛属; Tho: 索努斯属; Eud: 真矛属; Mic: 小矛线属; Pun: 螫属; Mes1: 中矛属; Lai: 咽针属; Apo: 孔咽属。
Fig. 3 Principal component analysis (PCA) of the nematode community. SWC, Soil water content; DOC, Dissolved organic carbon; DON, Dissolved organic nitrogen. The meaning of the symbol is shown in fig. 2. Black, green, purple and blue symbols represent 0, 15, 30 and 45 days of recovery after drought, respectively. Hollow indicates control, solid indicates drought. Abbreviations correspond to the nematode taxa were listed: Tyl1, Tylenchus; Mal, Malenchus; Psi, Psilenchus; Agl, Aglenchus; Fil, Filenchus; Cos, Coslenchus; Lel, Lelenchus; Bol, Boleodorus; Par, Paratylenchus; Neo, Neothada; Pra, Pratylenchus; Tyl2, Tylenchorhynchus; Cri, Criconemoides; Mel, Meloidogyne; Hel, Helicotylenchus; Lon, Longidorus; Xip, Xiphinema; Rha, Rhabditis; Mes2, Mesorhabditis; Odo, Odontolaimus; Gla, Glauxinema; Acr1, Acrobeles; Acr2, Acrobeloides; Cep, Cephalobus; Euc, Eucephalobus; Ple, Plectus; Ana, Anaplectus; Chi, Chiloplacus; Cer, Cervidellus; Chr, Chronogaster; Bas, Bastiania; Pri, Prismatolaimus; Ala, Alaimus; Amp, Amphidelus; Aph1, Aphelenchoides; Aph2, Aphelenchus; Dit, Ditylenchus, Dip, Diphtherophora; Tyl3, Tylencholaimus; Tri, Tripyla; Myl, Mylonchulus; Ach, Achromadora; Tho, Thonus; Eud, Eudorylaimus; Mic, Microdorylaimus; Pun, Pungentus; Mes1, Mesodorylaimus; Lai, Laimydorus; Apo, Aporcelaimellus.
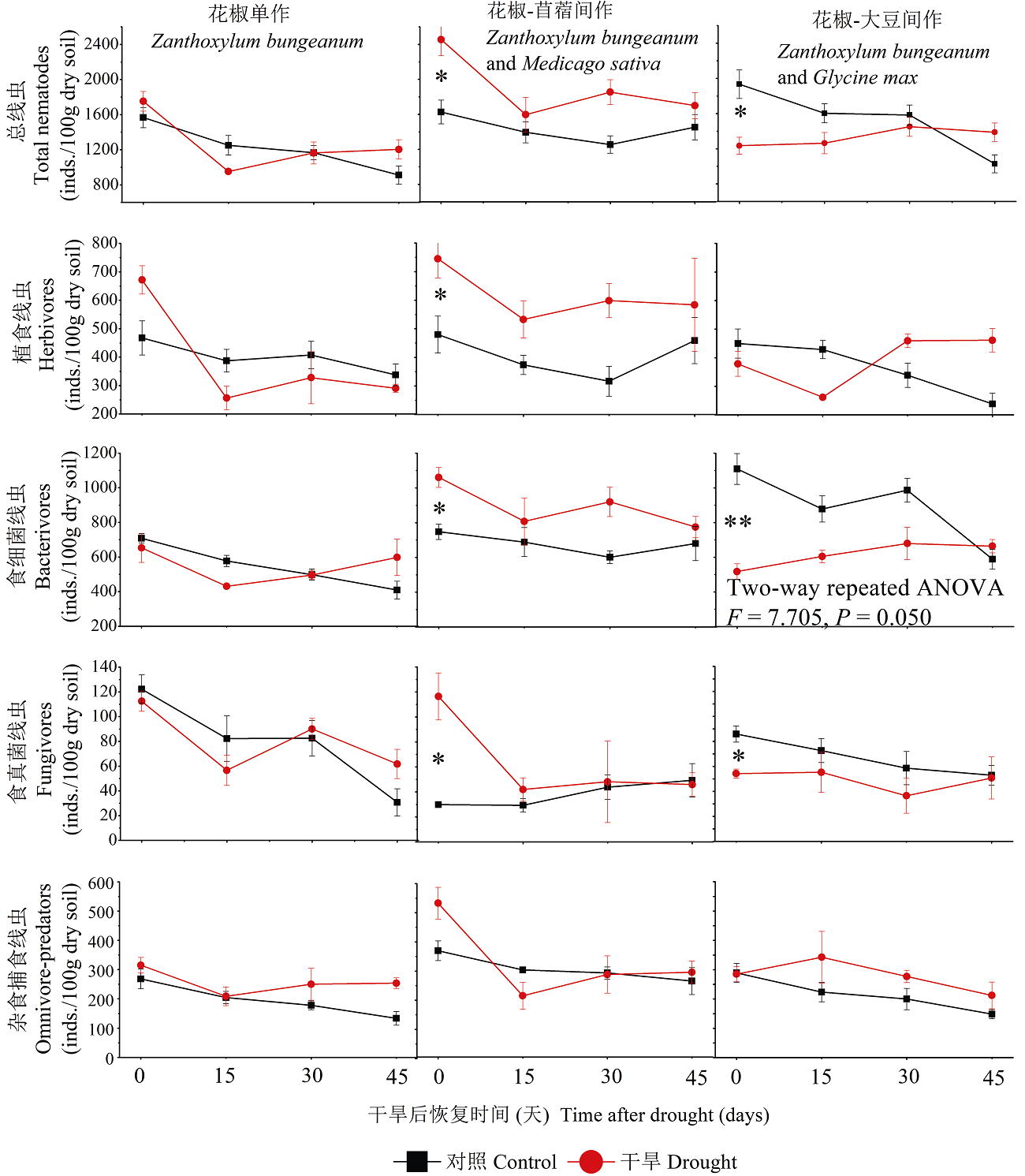
图4 不同种植模式下干旱后线虫营养类群。* P< 0.05, ** P< 0.01。
Fig. 4 Effects of planting systems on the nematode community after 45 days recovery from the drought. * P< 0.05, ** P< 0.01.
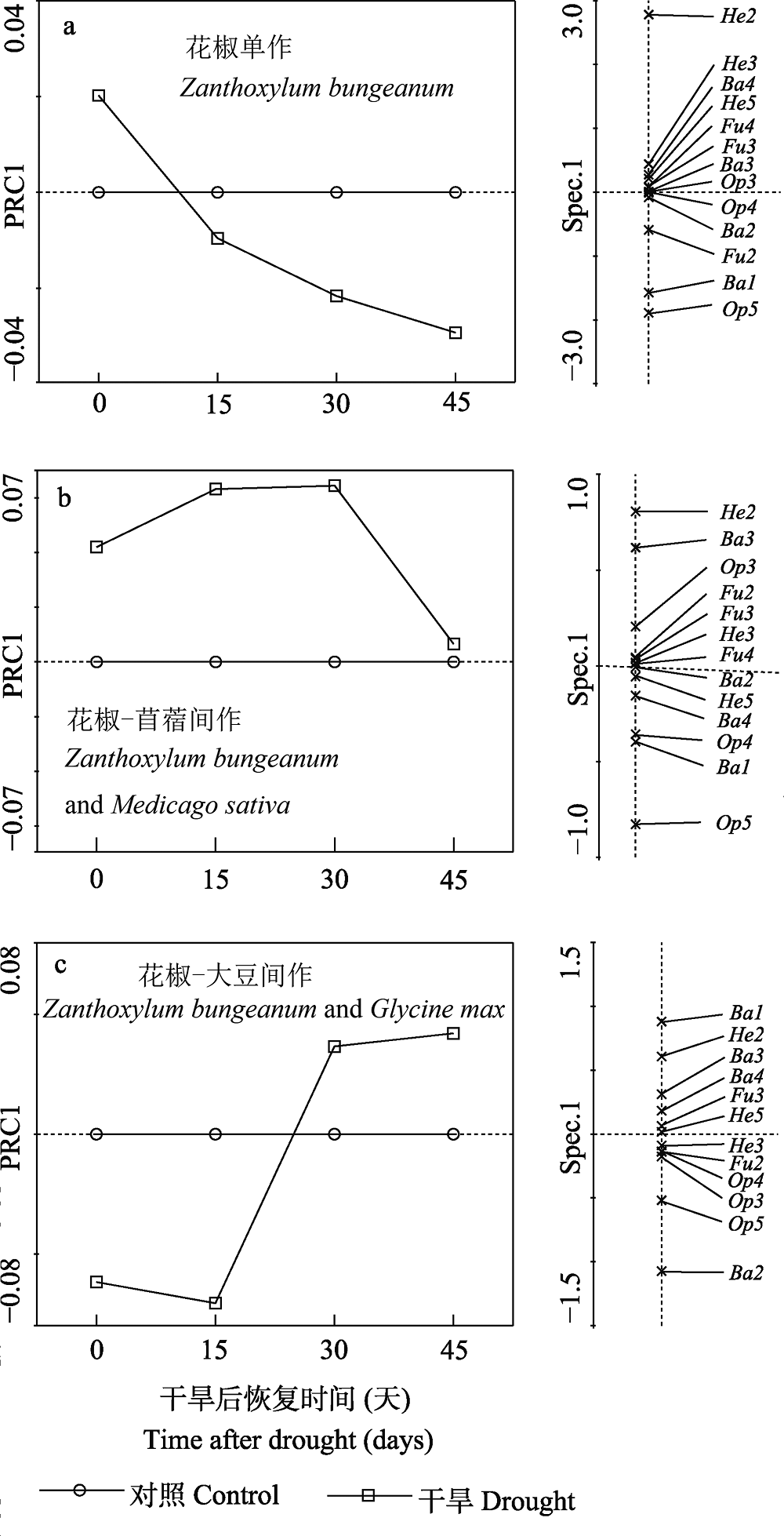
图5 主效应曲线分析干旱后不同种植模式下线虫功能团。PRC1代表对照处理,Ba: 食细菌线虫; Fu: 食真菌线虫; Op: 杂食捕食线虫; He: 植食性线虫, 数字1, 2, 3, 4和5代表线虫属的cp值。
Fig. 5 Principal response curves with weights of density of each soil nematode functional guild under different planting systems in control and drought. The horizontal axis represents the control treatment, Ba, Bacterivores; Fu, Fungivores; Op, Omnivore-predators; He, Herbivores. The number of 1, 2, 3, 4 and 5 are cp value of nematode genera.
| [1] |
Alster CJ, German DP, Lu Y, Allison SD (2013) Microbial enzymatic responses to drought and to nitrogen addition in a southern California grassland. Soil Biology and Biochemistry, 64, 68-79.
DOI URL |
| [2] |
Anderson SH, Udawatta RP, Seobi T, Garrett HE (2009) Soil water content and infiltration in agroforestry buffer strips. Agroforestry Systems, 75, 5-16.
DOI URL |
| [3] |
Andrés P, Moore JC, Simpson RT, Selby G, Cotrufo F, Denef K, Haddix ML, Shaw EA de Tomasel CM, Molowny-Horas R, Wall DH (2016) Soil food web stability in response to grazing in a semi-arid prairie: The importance of soil textural heterogeneity. Soil Biology and Biochemistry, 97, 131-143.
DOI URL |
| [4] |
Bertness MD, Callaway R (1994) Positive interactions in communities. Trends in Ecology and Evolution, 9, 191-193.
DOI PMID |
| [5] | Bongers T (1988) De Nematoden van Nederland. Pirola, Schoorl, the Netherlands. |
| [6] |
Borken W, Savage K, Davidson EA, Trumbore SE (2006) Effects of experimental drought on soil respiration and radiocarbon efflux from a temperate forest soil. Global Change Biology, 12, 177-193.
DOI URL |
| [7] |
Bossio DA, Scow KM (1998) Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Microbial Ecology, 35, 265-278.
PMID |
| [8] |
Burgess SSO, Adams MA, Turner NC, White DA, Ong CK (2001) Tree roots: Conduits for deep recharge of soil water. Oecologia, 126, 158-165.
DOI URL |
| [9] |
Dai AG (2013) Increasing drought under global warming in observations and models. Nature Climate Change, 3, 52-58.
DOI URL |
| [10] |
Daryanto S, Wang LX, Jacinthe PA (2017) Global synthesis of drought effects on cereal, legume, tuber and root crops production: A review. Agricultural Water Management, 179, 18-33.
DOI URL |
| [11] |
de Vries FT, Liiri ME, Bjørnlund L, Bowker MA, Christensen S, Setälä HM, Bardgett RD (2012) Land use alters the resistance and resilience of soil food webs to drought. Nature Climate Change, 2, 276-280.
DOI URL |
| [12] | de Vries FT, Shade A (2013) Controls on soil microbial community stability under climate change. Frontiers in Microbiology, 4, 1-16. |
| [13] |
Frostegård A, Tunlid A, Bååth E (1996) Changes in microbial community structure during long-term incubation in two soils experimentally contaminated with metals. Soil Biology and Biochemistry, 28, 55-63.
DOI URL |
| [14] |
Grant K, Kreyling J, Heilmeier H, Beierkuhnlein C, Jentsch A (2014) Extreme weather events and plant-plant interactions: Shifts between competition and facilitation among grassland species in the face of drought and heavy rainfall. Ecological Research, 29, 991-1001.
DOI URL |
| [15] |
Hueso S, García C, Hernández T (2012) Severe drought conditions modify the microbial community structure, size and activity in amended and unamended soils. Soil Biology and Biochemistry, 50, 167-173.
DOI URL |
| [16] | IUSS Working Group WRB (2007) World Soil Resources Reports no. 103. FAO, Rome. |
| [17] |
Khan MASA, Grant K, Beierkuhnlein C, Kreyling J, Jentsch A (2014) Climatic extremes lead to species-specific legume facilitation in an experimental temperate grassland. Plant and Soil, 379, 161-175.
DOI URL |
| [18] |
Kreyling J, Beierkuhnlein C, Elmer M, Pritsch K, Radovski M, Schloter M, Wöllecke J, Jentsch A (2008) Soil biotic processes remain remarkably stable after 100-year extreme weather events in experimental grassland and heath. Plant and Soil, 308, 175-188.
DOI URL |
| [19] | Lepš J, Šmilauer P (2003) Multivariate Analysis of Ecological Data using CANOCO. Cambridge University Press, New York. |
| [20] |
Lindberg N, Bengtsson J (2006) Recovery of forest soil fauna diversity and composition after repeated summer droughts. Oikos, 114, 494-506.
DOI URL |
| [21] |
Loreau M, Naeem S, Inchausti P, Bengtsson J, Grime JP, Hector A, Hooper DU, Huston MA, Raffaelli D, Schmid B, Tilman D, Wardle DA (2001) Biodiversity and ecosystem functioning: Current knowledge and future challenges. Science, 294, 804-808.
PMID |
| [22] |
Maraldo K, Holmstrup M (2009) Recovery of enchytraeid populations after severe drought events. Applied Soil Ecology, 42, 227-235.
DOI URL |
| [23] |
Narain P, Singh RK, Sindhwal NS, Joshie P (1998) Water balance and water use efficiency of different land uses in western Himalayan valley region. Agricultural Water Management, 37, 225-240.
DOI URL |
| [24] |
Orwin KH, Wardle DA (2005) Plant species composition effects on belowground properties and the resistance and resilience of the soil microflora to a drying disturbance. Plant and Soil, 278, 205-221.
DOI URL |
| [25] |
Papatheodorou EM, Kordatos H, Kouseras T, Monokrousos N, Menkissoglu-Spiroudi U, Diamantopoulos J, Stamou GP, Argyropoulou MD (2012) Differential responses of structural and functional aspects of soil microbes and nematodes to abiotic and biotic modifications of the soil environment. Applied Soil Ecology, 61, 26-33.
DOI URL |
| [26] |
Rivest D, Paquette A, Shipley B, Reich PB, Messier C (2015) Tree communities rapidly alter soil microbial resistance and resilience to drought. Functional Ecology, 29, 570-578.
DOI URL |
| [27] |
Sanaullah M, Blagodatskaya E, Chabbi A, Rumpel C, Kuzyakov Y (2011) Drought effects on microbial biomass and enzyme activities in the rhizosphere of grasses depend on plant community composition. Applied Soil Ecology, 48, 38-44.
DOI URL |
| [28] |
Shao YH, Fu SL (2007) The diversity and functions of soil nematodes. Biodiversity Science, 15, 116-123. (in Chinese with English abstract)
DOI URL |
|
[邵元虎, 傅声雷 (2007) 试论土壤线虫多样性在生态系统中的作用. 生物多样性, 15, 116-123.]
DOI |
|
| [29] | Shi LL, Fu SL (2014) Review of soil biodiversity research: History, current status and future challenges. Chinese Science Bulletin, 59, 493-509. (in Chinese with English abstract) |
| [时雷雷, 傅声雷 (2014) 土壤生物多样性研究: 历史、现状与挑战. 科学通报, 59, 493-509.] | |
| [30] |
Stevnbak K, Scherber C, Gladbach DJ, Beier C, Mikkelsen TN, Christensen S (2012) Interactions between above- and belowground organisms modified in climate change experiments. Nature Climate Change, 2, 805-808.
DOI URL |
| [31] |
Sun F, Pan KW, Tariq A, Zhang L, Sun XM, Li ZL, Wang SZ, Xiong QL, Song DG, Olatunji OA (2016) The response of the soil microbial food web to extreme rainfall under different plant systems. Scientific Reports, 6, 37662.
DOI URL |
| [32] |
Townshend JL (1963) A modification and evaluation of the apparatus for the oostenbrink direct cottonwool filter extraction method. Nematologica, 9, 106-110.
DOI URL |
| [33] |
Viketoft M, Bengtsson J, Sohlenius B, Berg MP, Petchey O, Palmborg C, Huss-Danell K (2009) Long-term effects of plant diversity and composition on soil nematode communities in model grasslands. Ecology, 90, 90-99.
PMID |
| [34] |
Wallenstein MD, Hall EK (2012) A trait-based framework for predicting when and where microbial adaptation to climate change will affect ecosystem functioning. Biogeochemistry, 109, 35-47.
DOI URL |
| [35] |
Wang ZY, Silva LCR, Sun G, Luo P, Mou CX, Horwath WR (2015) Quantifying the impact of drought on soil-plant interactions: A seasonal analysis of biotic and abiotic controls of carbon and nutrient dynamics in high-altitudinal grasslands. Plant and Soil, 389, 59-71.
DOI URL |
| [36] | Xu K, Yang DW, Yang HB, Li Z, Qin Y, Shen Y (2015) Spatio-temporal variation of drought in China during 1961-2012: A climatic perspective. Journal of Hydrology, 526, 253-264. |
| [37] |
Zhao J, Wang XL, Wang XL, Fu SL (2014) Legume-soil interactions: Legume addition enhances the complexity of the soil food web. Plant and Soil, 385, 273-286.
DOI URL |
| [1] | 褚晓琳, 张全国. 演化速率假说的实验验证研究进展[J]. 生物多样性, 2025, 33(4): 25019-. |
| [2] | 宋威, 程才, 王嘉伟, 吴纪华. 土壤微生物对植物多样性–生态系统功能关系的调控作用[J]. 生物多样性, 2025, 33(4): 24579-. |
| [3] | 莫笑梅, 张琪, 杨嘉欣, 郑国, 胡中民, 张晓珂, 梁思维, 崔淑艳. 北方典型草地土壤线虫代谢速率及能量流动对氮沉降和降水模式改变的响应[J]. 生物多样性, 2025, 33(3): 24341-. |
| [4] | 刘淑琪, 崔东, 江智诚, 刘江慧, 闫江超. 短期氮、水添加和刈割减弱了苦豆子型退化草地土壤生物多样性与生态系统多功能性的联系[J]. 生物多样性, 2025, 33(3): 24305-. |
| [5] | 刘源, 杜剑卿, 马丽媛, 杨刚, 田建卿. 纳木措流域岸边带湿地产甲烷古菌群落多样性与分布特征[J]. 生物多样性, 2025, 33(1): 24247-. |
| [6] | 陈楠, 张全国. 实验进化研究途径[J]. 生物多样性, 2024, 32(9): 24171-. |
| [7] | 连佳丽, 陈婧, 杨雪琴, 赵莹, 罗叙, 韩翠, 赵雅欣, 李建平. 荒漠草原植物多样性和微生物多样性对降水变化的响应[J]. 生物多样性, 2024, 32(6): 24044-. |
| [8] | 姚祝, 魏雪, 马金豪, 任晓, 王玉英, 胡雷, 吴鹏飞. 气候暖湿化对高寒草甸土壤线虫群落的短期影响[J]. 生物多样性, 2024, 32(5): 23483-. |
| [9] | 郝操, 吴东辉, 莫凌梓, 徐国良. 越冬动物肠道微生物多样性及功能研究进展[J]. 生物多样性, 2024, 32(3): 23407-. |
| [10] | 曹可欣, 王敬雯, 郑国, 武鹏峰, 李英滨, 崔淑艳. 降水格局改变及氮沉降对北方典型草原土壤线虫多样性的影响[J]. 生物多样性, 2024, 32(3): 23491-. |
| [11] | 赵榕江, 吴纪华, 何维明, 赵彩云, 周波, 李博, 杨强. 土壤生物多样性与外来植物入侵: 进展与展望[J]. 生物多样性, 2024, 32(11): 24243-. |
| [12] | 杨舒涵, 王贺, 陈磊, 廖蓥飞, 严光, 伍一宁, 邹红菲. 松嫩平原异质生境对土壤线虫群落特征的影响[J]. 生物多样性, 2024, 32(1): 23295-. |
| [13] | 罗正明, 刘晋仙, 张变华, 周妍英, 郝爱华, 杨凯, 柴宝峰. 不同退化阶段亚高山草甸土壤原生生物群落多样性特征及驱动因素[J]. 生物多样性, 2023, 31(8): 23136-. |
| [14] | 吴春玲, 罗竹慧, 李意德, 许涵, 陈德祥, 丁琼. 热带山地雨林木本豆科和樟科植物叶内生细菌群落: 物种与功能群多样性及驱动因子[J]. 生物多样性, 2023, 31(8): 23146-. |
| [15] | 朱晓华, 高程, 王聪, 赵鹏. 尿素对土壤细菌与真菌多样性影响的研究进展[J]. 生物多样性, 2023, 31(6): 22636-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
![]()
