



生物多样性 ›› 2025, Vol. 33 ›› Issue (1): 24247. DOI: 10.17520/biods.2024247 cstr: 32101.14.biods.2024247
刘源1,2, 杜剑卿3,4( ), 马丽媛4, 杨刚1,*(
), 马丽媛4, 杨刚1,*( )(
)( ), 田建卿2,*(
), 田建卿2,*( )(
)( )
)
收稿日期:2024-06-19
接受日期:2024-09-24
出版日期:2025-01-20
发布日期:2024-12-19
通讯作者:
* E-mail: 基金资助:
Yuan Liu1,2, Jianqing Du3,4( ), Liyuan Ma4, Gang Yang1,*(
), Liyuan Ma4, Gang Yang1,*( )(
)( ), Jianqing Tian2,*(
), Jianqing Tian2,*( )(
)( )
)
Received:2024-06-19
Accepted:2024-09-24
Online:2025-01-20
Published:2024-12-19
Contact:
* E-mail: Supported by:摘要:
高寒河流型湿地生态系统是甲烷排放的重要区域。产甲烷古菌是湿地生境中甲烷产生的主要来源之一, 其群落组成变化显著影响全球碳循环过程。然而, 高寒河流型湿地产甲烷古菌群落的组成与分布特征尚不明确。因此, 本研究以青藏高原纳木措湖尼亚曲流域为研究对象, 利用mcrA基因扩增子测序技术, 对横向(岸边带湿地、过渡带、高寒草甸)和纵向(4,980 m、4,843 m、4,777 m、4,752 m 4个海拔梯度)两个维度上的土壤产甲烷古菌进行分析, 探讨其多样性、群落结构及分布模式。结果表明, 从岸边带湿地至过渡带再到高寒草甸, 产甲烷古菌群落的α多样性逐渐降低, 岸边带湿地群落组成显著不同于高寒草甸和过渡带。在所有样点中, 氢营养型甲烷杆菌属(Methanobacterium)是最主要的产甲烷古菌(高寒草甸、过渡带和岸边带湿地的平均相对丰度依次为45.78%、42.90%及34.17%)。中性群落模型表明, 随机过程是岸边带湿地产甲烷古菌群落构建的主要驱动因素, 但随机过程对高寒草甸及过渡带群落贡献较少。FEAST溯源分析表明, 横向维度上, 高寒草甸和过渡带对岸边带湿地产甲烷古菌群落的贡献率分别为17.62%和13.04%; 纵向维度上, 低海拔(样点S4)岸边带湿地产甲烷古菌群落主要由河流上游岸边带湿地(49.71%)和高寒草甸(21.45%)输入, 表明高寒草甸是岸边带湿地产甲烷古菌群落的重要物种库。本研究揭示了高寒流域土壤产甲烷古菌群落的多样性组成及分布模式, 对理解高寒生态系统功能具有重要意义。
刘源, 杜剑卿, 马丽媛, 杨刚, 田建卿 (2025) 纳木措流域岸边带湿地产甲烷古菌群落多样性与分布特征. 生物多样性, 33, 24247. DOI: 10.17520/biods.2024247.
Yuan Liu, Jianqing Du, Liyuan Ma, Gang Yang, Jianqing Tian (2025) Diversity and distribution of methanogen communities in the riparian wetlands of the Nam Co basin. Biodiversity Science, 33, 24247. DOI: 10.17520/biods.2024247.
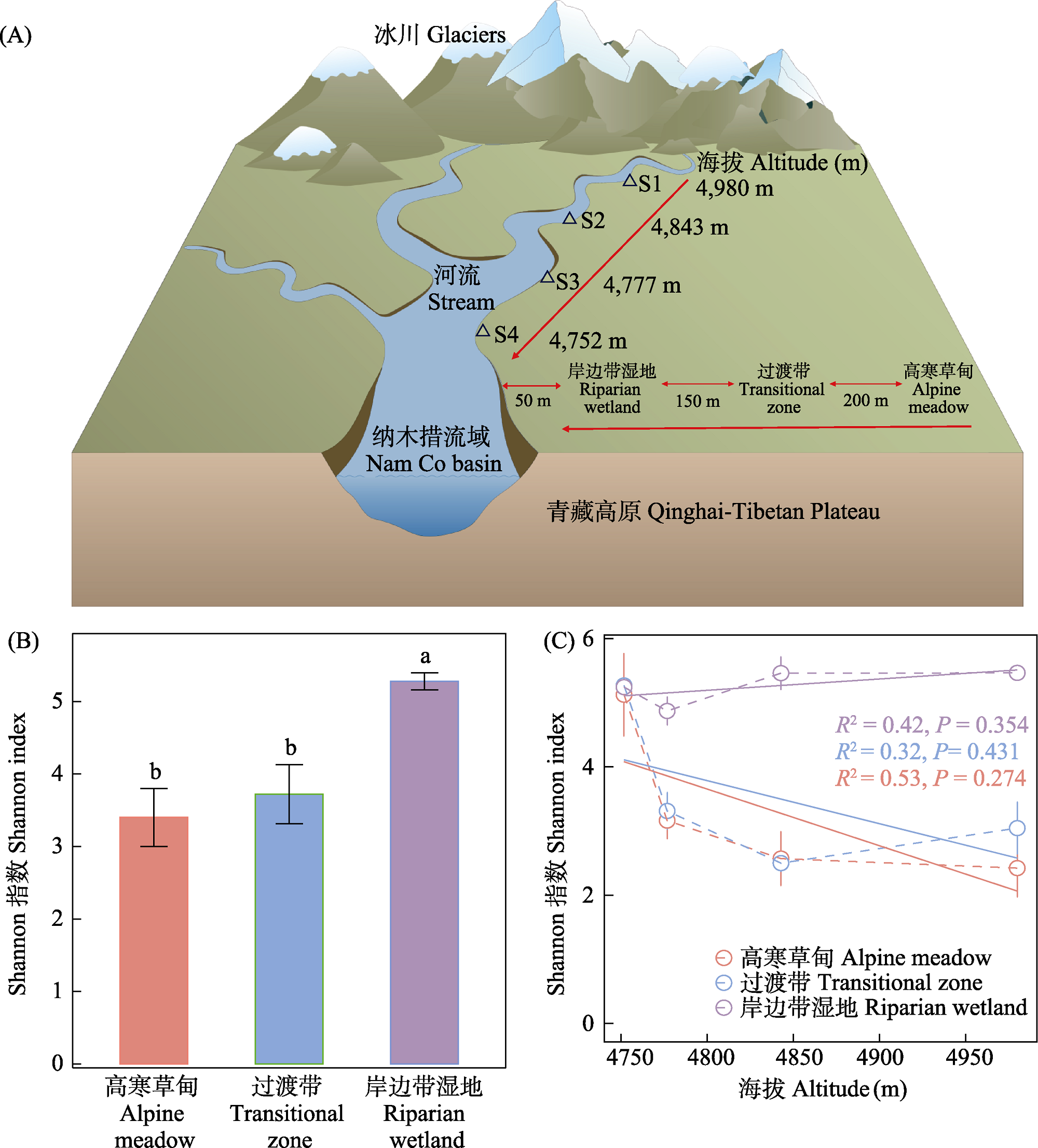
图1 不同生态类型土壤样品采集及产甲烷古菌群落α多样性。(A)青藏高原纳木措湖尼亚曲流域采样示意图; (B)不同类型土壤产甲烷古菌Shannon指数差异(平均值 ± 标准误); (C)不同土壤类型产甲烷古菌Shannon指数随海拔梯度的变化(平均值 ± 标准误)。不同字母表示差异显著(P < 0.05)。S1-S4表示样地。
Fig. 1 Sample collection and α-diversity of methanogen communities in soils of different ecological types. (A) Schematic diagram of sampling locations in the Niyaqu basin of Nam Co basin on the Qinghai-Tibetan Plateau; (B) Differences in the Shannon index of methanogens across different soil types (mean ± SE); (C) Variations in the Shannon index of methanogens in different soil types along the altitudinal gradient (mean ± SE). Different letters indicate significant differences (P < 0.05). S1-S4 indicate sample plots.
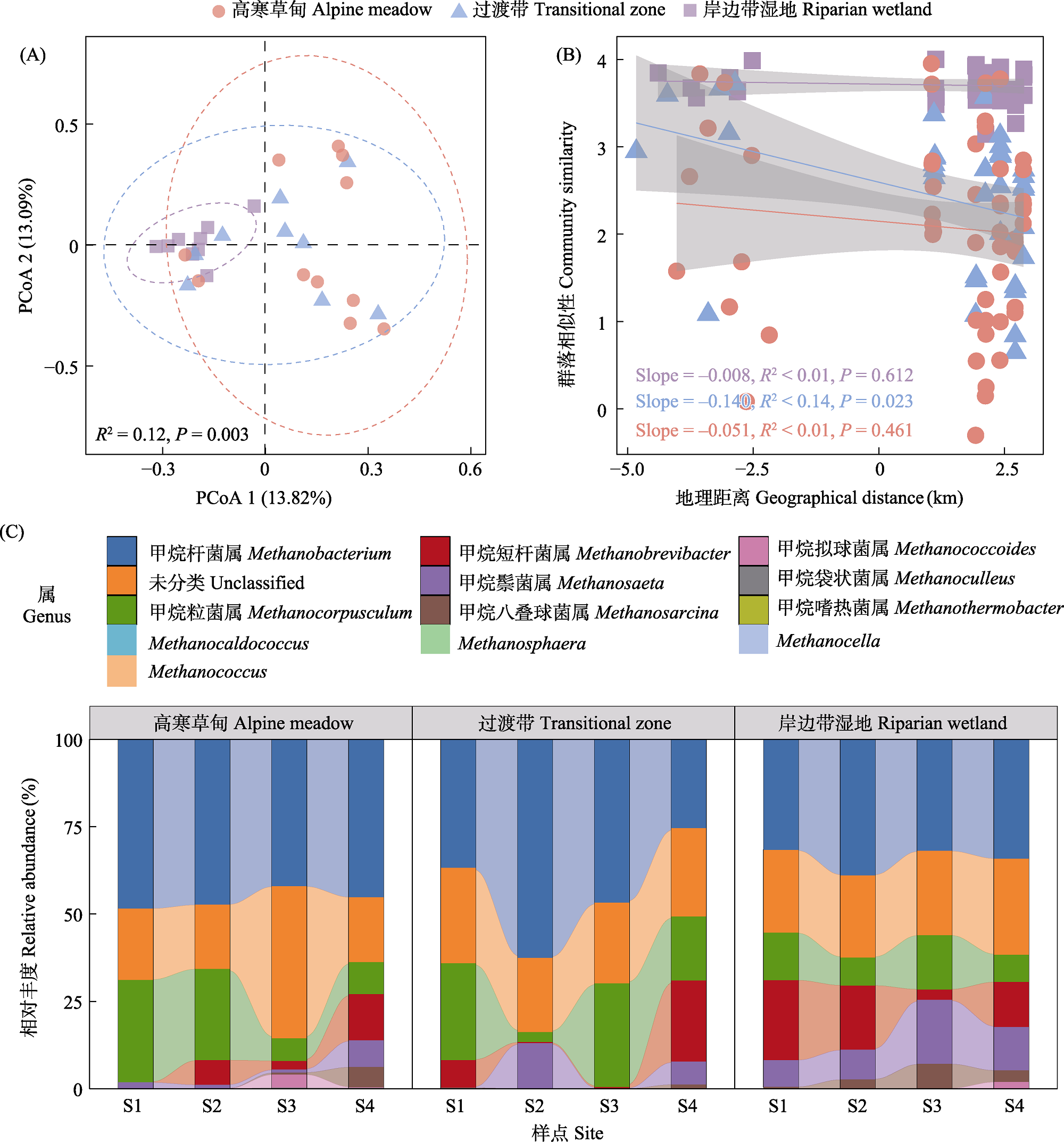
图2 产甲烷古菌群落结构、组成变化以及距离衰减关系分析。(A)基于Bray-Curtis距离的主坐标分析(PCoA)显示产甲烷古菌群落的结构变化; (B)距离衰减曲线显示Bray-Curtis相似性与采样点之间地理距离的关系(数据经过自然对数转换); (C)不同土壤类型各样点产甲烷古菌群落组成和相对丰度的变化。图A中的虚线椭圆和图B中的阴影区域表示95%的置信区间, 图B中的实线表示普通最小二乘法线性回归。
Fig. 2 Analyses of methanogen community structure, compositional changes, and distance-decay relationships. (A) Differences in methanogen communities based on Bray-Curtis distances; (B) Distance-decay curve showing the relationship between Bray-Curtis similarity and geographical distance (data converted to natural logarithm); (C) Methanogens composition at each sample site across different soil types. The dashed ellipse in Figure A and the shaded area in Figure B indicate 95% confidence intervals, and the solid line in Figure (B) indicates ordinary least squares linear regression.
| 类型 Type | R2 | P |
|---|---|---|
| 高寒草甸 vs. 过渡带 Alpine meadow/Transitional zone | 0.055 | 0.353 |
| 高寒草甸 vs. 岸边带湿地 Alpine meadow/Riparian wetland | 0.117 | 0.001 |
| 过渡带 vs. 岸边带湿地 Transitional zone/Riparian wetland | 0.105 | 0.004 |
表1 不同生态类型土壤产甲烷古菌群落结构差异的置换多元方差分析
Table 1 Permutational multivariate analysis of variance of methanogen community structure differences in soils of different ecological types
| 类型 Type | R2 | P |
|---|---|---|
| 高寒草甸 vs. 过渡带 Alpine meadow/Transitional zone | 0.055 | 0.353 |
| 高寒草甸 vs. 岸边带湿地 Alpine meadow/Riparian wetland | 0.117 | 0.001 |
| 过渡带 vs. 岸边带湿地 Transitional zone/Riparian wetland | 0.105 | 0.004 |
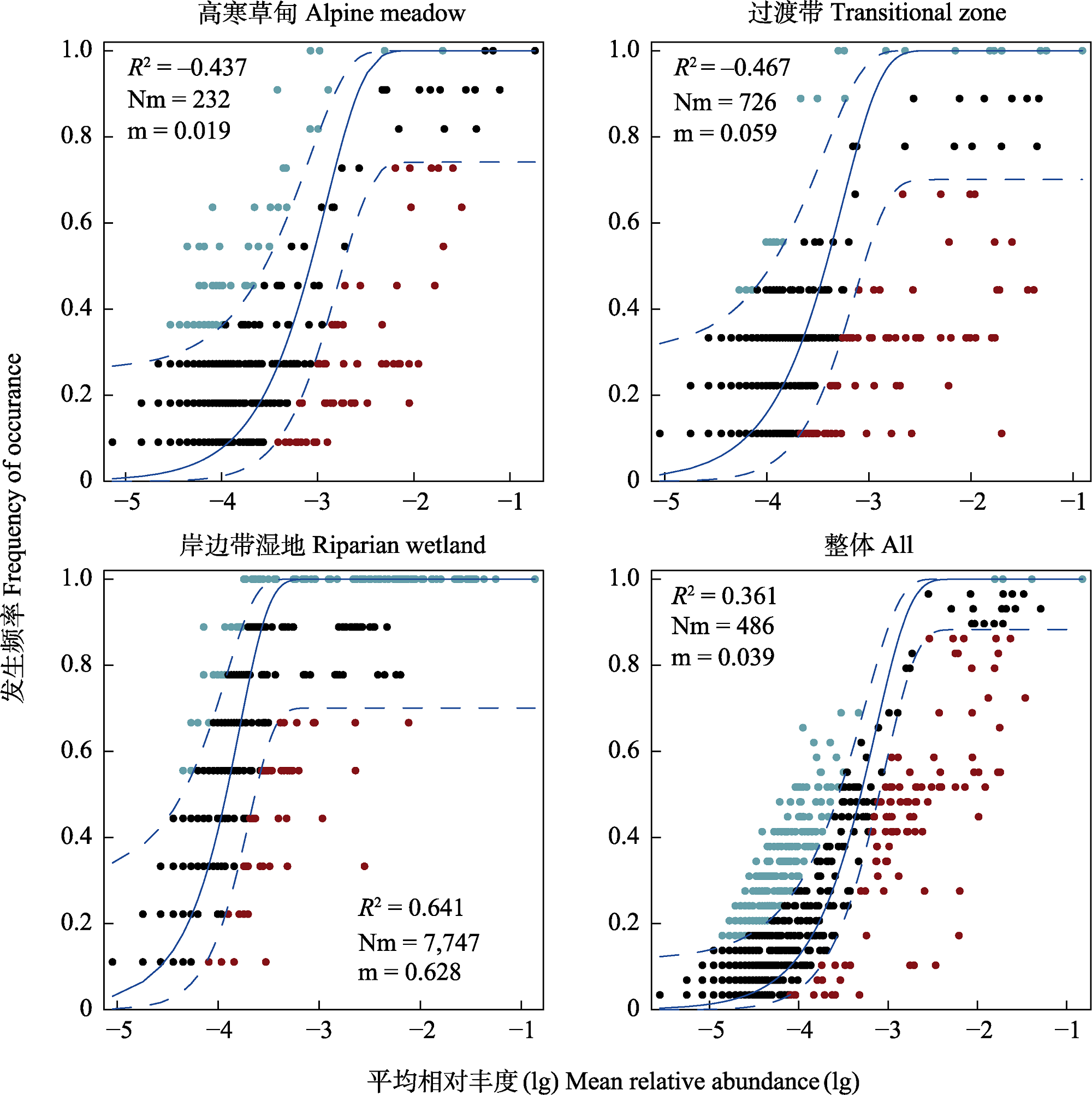
图3 中性群落模型拟合不同土壤类型产甲烷古菌群落中OTUs的出现频率与相对丰度之间的关系。R2代表了中性群落模型的整体拟合优度, 负R2值表明中性群落模型拟合不佳。Nm是元群落规模(N)与迁移率(m)的乘积。图中蓝色实线表示中性群落模型的最适拟合值, 蓝色虚线代表模型的95%置信区间(通过1,000次bootstrap获得估计), 绿色表示出现频率高于中性群落模型预测的OTUs, 红色表示出现频率高于中性群落模型预测的OTUs。
Fig. 3 The neutral community model fits the relationship between the frequency and relative abundance of OTUs in methanogenic archaeal communities in different soil types. R2 represents the overall goodness of fit of the neutral community model, with a negative R2 indicating a poor fit of the neutral community model. Nm is the product of the metacommunity size (N) and the mobility (m). The solid blue line in the figure represents the best-fit value of the neutral community model, the blue dashed line represents the 95% confidence interval of the model (estimate obtained by 1000 bootstrap), green indicates OTUs that occur more frequently than predicted by the neutral community model, and red indicates OTUs that occur more frequently than predicted by the neutral community model.
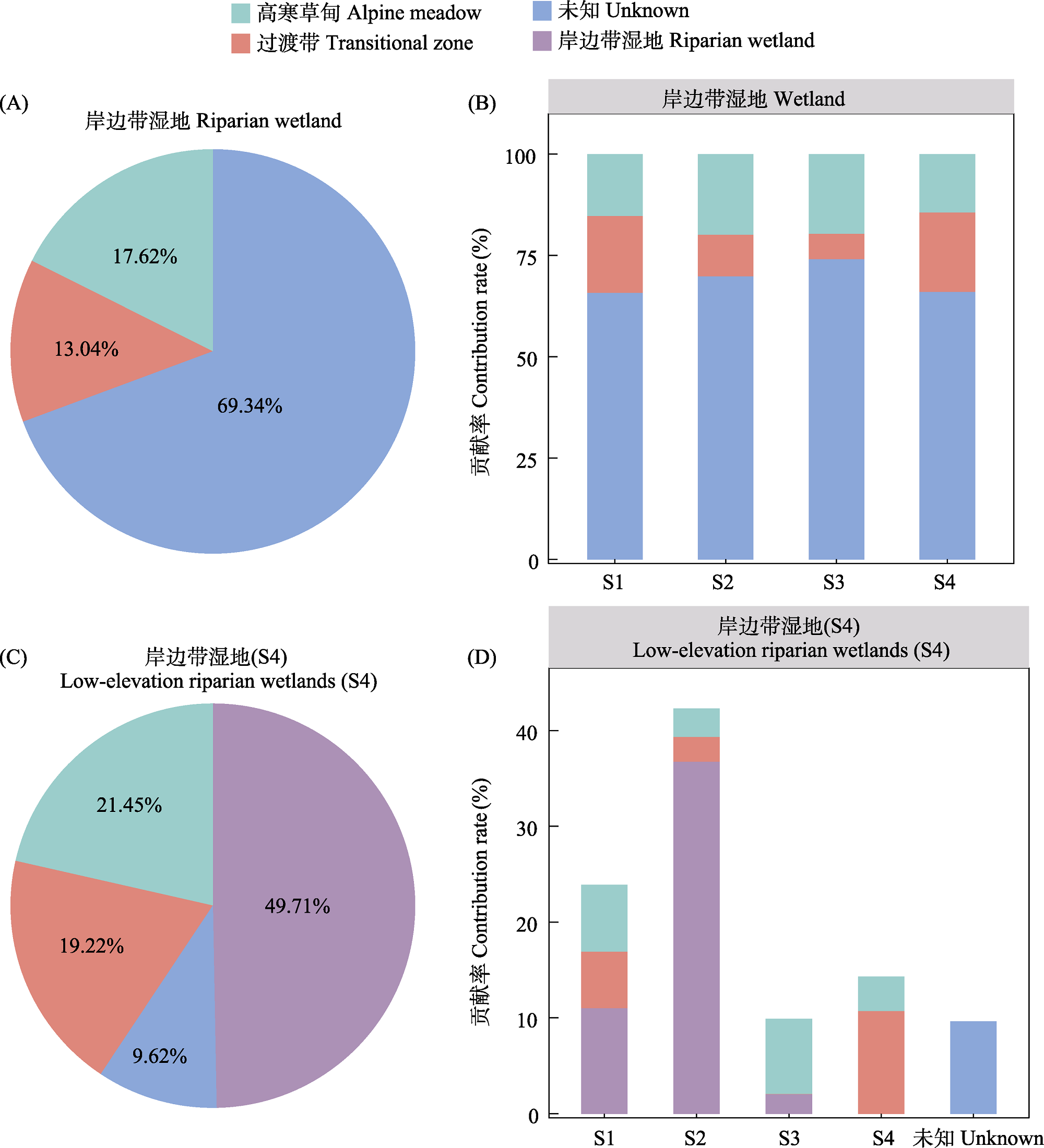
图4 不同源群落对岸边带湿地(横向和纵向)产甲烷古菌群落的贡献。(A)高寒草甸和过渡带土壤的微生物群落对岸边带湿地整体贡献度; (B)各样点中岸边带湿地群落的来源分析; (C)来自3个不同生态类型土壤的产甲烷古菌群落对低海拔岸边带湿地(S4)群落的整体贡献度; (D)各样点中3种生态类型土壤群落对低海拔岸边带湿地(S4)群落的贡献。
Fig. 4 Contribution of different source communities to methanogen communities in riparian wetlands (lateral and longitudinal dimensions). (A) Overall contribution of microbial communities from alpine meadows and transition zone soils to riparian wetlands; (B) Source analysis of riparian wetland communities at each sample site; (C) Overall contribution of methanogen communities in soils from the three different ecological types to the terminal riparian wetland (S4) community; (D) Contribution of the three soil community types to the terminal riparian wetland (S4) community at each site.
| [1] | Albright MBN, Martiny JBH (2018) Dispersal alters bacterial diversity and composition in a natural community. The ISME Journal, 12, 296-299. |
| [2] |
Anslan S, Azizi Rad M, Buckel J, Echeverria Galindo P, Kai JL, Kang WG, Keys L, Maurischat P, Nieberding F, Reinosch E, Tang HD, Tran TV, Wang YY, Schwalb A (2020) Reviews and syntheses: How do abiotic and biotic processes respond to climatic variations in the Nam Co catchment (Tibetan Plateau)? Biogeosciences, 17, 1261-1279.
DOI |
| [3] |
Argiroff WA, Zak DR, Lanser CM, Wiley MJ (2017) Microbial community functional potential and composition are shaped by hydrologic connectivity in riverine floodplain soils. Microbial Ecology, 73, 630-644.
DOI PMID |
| [4] |
Blake DR, Rowland FS (1988) Continuing worldwide increase in tropospheric methane, 1978 to 1987. Science, 239, 1129-1131.
PMID |
| [5] |
Bolger AM, Lohse M, Usadel B (2014) Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics, 30, 2114-2120.
DOI PMID |
| [6] | Bräuer SL, Basiliko N, Siljanen HMP, Zinder SH (2020) Methanogenic Archaea in peatlands. FEMS Microbiology Letters, 367, fnaa172. |
| [7] | Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecology, 85, 1771-1789. |
| [8] | Cao MM, Wang F, Ma S, Geng HH, Sun K (2024) Recent advances on greenhouse gas emissions from wetlands: Mechanism, global warming potential, and environmental drivers. Environmental Pollution, 355, 124204. |
| [9] |
Carlson BS, Rotics S, Nathan R, Wikelski M, Jetz W (2021) Individual environmental niches in mobile organisms. Nature Communications, 12, 4572.
DOI PMID |
| [10] |
Chase JM (2010) Stochastic community assembly causes higher biodiversity in more productive environments. Science, 328, 1388-1391.
DOI PMID |
| [11] | Chen HB, Chang S (2020) Dissecting methanogenesis for temperature-phased anaerobic digestion: Impact of temperature on community structure, correlation, and fate of methanogens. Bioresource Technology, 306, 123104. |
| [12] |
Chen SZ, Wang P, Liu HD, Xie W, Wan XS, Kao SJ, Phelps TJ, Zhang CL (2020) Population dynamics of methanogens and methanotrophs along the salinity gradient in Pearl River Estuary: Implications for methane metabolism. Applied Microbiology and Biotechnology, 104, 1331-1346.
DOI PMID |
| [13] | Cheng L, Zheng ZZ, Wang C, Zhang H (2016) Recent advances in methanogens. Microbiology China, 43, 1143-1164. (in Chinese with English abstract) |
| [承磊, 郑珍珍, 王聪, 张辉 (2016) 产甲烷古菌研究进展. 微生物学通报, 43, 1143-1164.] | |
| [14] | Conrad R (2007) Microbial ecology of methanogens and methanotrophs. Advances in Agronomy, 96, 1-63. |
| [15] | Crump BC, Amaral-Zettler LA, Kling GW (2012) Microbial diversity in Arctic freshwaters is structured by inoculation of microbes from soils. The ISME Journal, 6, 1629-1639. |
| [16] | Dini-Andreote F, Stegen JC, van Elsas JD, Salles JF (2015) Disentangling mechanisms that mediate the balance between stochastic and deterministic processes in microbial succession. Proceedings of the National Academy of Sciences, USA, 112, E1326-E1332. |
| [17] |
Finn DR, Ziv-El M, van Haren J, Park JG, Del Aguila-Pasquel J, Urquiza-Muñoz JD, Cadillo-Quiroz H (2020) Methanogens and methanotrophs show nutrient-dependent community assemblage patterns across tropical peatlands of the Pastaza-marañón basin, Peruvian Amazonia. Frontiers in Microbiology, 11, 746.
DOI PMID |
| [18] | Fuhrman JA, Steele JA, Hewson I, Schwalbach MS, Brown MV, Green JL, Brown JH (2008) A latitudinal diversity gradient in planktonic marine bacteria. Proceedings of the National Academy of Sciences, USA, 105, 7774-7778. |
| [19] |
Galand PE, Fritze H, Conrad R, Yrjälä K (2005) Pathways for methanogenesis and diversity of methanogenic Archaea in three boreal peatland ecosystems. Applied and Environmental Microbiology, 71, 2195-2198.
PMID |
| [20] | Gao TG, Kang SC, Zhang TJ, Zhou SQ, Cuo L, Sillanpää M, Zhang YL (2015) Summer hydrological characteristics in glacier and non-glacier catchments in the Nam Co Basin, southern Tibetan Plateau. Environmental Earth Sciences, 74, 2019-2028. |
| [21] | Gu H, Xiao FS, He ZL, Yan QY (2018) Microbial driven methane emission mechanisms in wetland ecosystems. Acta Microbiologica Sinica, 58, 618-632. (in Chinese with English abstract) |
| [顾航, 肖凡书, 贺志理, 颜庆云 (2018) 湿地微生物介导的甲烷排放机制. 微生物学报, 58, 618-632.] | |
| [22] |
Hattori S (2008) Syntrophic acetate-oxidizing microbes in methanogenic environments. Microbes and Environments, 23, 118-127.
PMID |
| [23] | Heino J, Melo AS, Siqueira T, Soininen J, Valanko S, Bini LM (2015) Metacommunity organisation, spatial extent and dispersal in aquatic systems: Patterns, processes and prospects. Freshwater Biology, 60, 845-869. |
| [24] | Huber P, Metz S, Unrein F, Mayora G, Sarmento H, Devercelli M (2020) Environmental heterogeneity determines the ecological processes that govern bacterial metacommunity assembly in a floodplain river system. The ISME Journal, 14, 2951-2966. |
| [25] | Hui CZ, Li Y, Yuan SY, Zhang WL (2023) River connectivity determines microbial assembly processes and leads to alternative stable states in river networks. Science of the Total Environment, 904, 166797. |
| [26] | Hui CZ, Li Y, Zhang WL, Zhang C, Niu LH, Wang LF, Zhang HJ (2022) Modelling structure and dynamics of microbial community in aquatic ecosystems: The importance of hydrodynamic processes. Journal of Hydrology, 605, 127351. |
| [27] |
Immerzeel WW, van Beek LPH, Bierkens MFP (2010) Climate change will affect the Asian water towers. Science, 328, 1382-1385.
DOI PMID |
| [28] | Iqbal A, Shang ZH, Rehman MLU, Ju MT, Rehman MMU, Rafiq MK, Ayub N, Bai YF (2019) Pattern of microbial community composition and functional gene repertoire associated with methane emission from Zoige wetlands, China—A review. Science of the Total Environment, 694, 133675. |
| [29] | Kaiser K, Miehe G, Barthelmes A, Ehrmann O, Scharf A, Schult M, Schlütz F, Adamczyk S, Frenzel B (2008) Turf-bearing topsoils on the central Tibetan Plateau, China: Pedology, botany, geochronology. Catena, 73, 300-311. |
| [30] |
Kotsyurbenko OR (2005) Trophic interactions in the methanogenic microbial community of low-temperature terrestrial ecosystems. FEMS Microbiology Ecology, 53, 3-13.
PMID |
| [31] | Lear G, Bellamy J, Case BS, Lee JE, Buckley HL (2014) Fine-scale spatial patterns in bacterial community composition and function within freshwater ponds. The ISME Journal, 8, 1715-1726. |
| [32] | Leibold MA, Holyoak M, Mouquet N, Amarasekare P, Chase JM, Hoopes MF, Holt RD, Shurin JB, Law R, Tilman D, Loreau M, Gonzalez A (2004) The metacommunity concept: A framework for multi-scale community ecology. Ecology Letters, 7, 601-613. |
| [33] | Leng H, Yang Q, Huang GF, Bai LP (2020) Recent advances in hydrogenotrophic methanogenesis. Acta Microbiologica Sinica, 60, 2136-2160. (in Chinese with English abstract) |
| [冷欢, 杨清, 黄钢锋, 白丽萍 (2020) 氢营养型产甲烷代谢途径研究进展. 微生物学报, 60, 2136-2160.] | |
| [34] | Li CN, Tu B, Kou YP, Wang YS, Li XZ, Wang JM, Li JB (2021) The assembly of methanotrophic communities regulated by soil pH in a mountain ecosystem. Catena, 196, 104883. |
| [35] | Li TT, Lu YY, Yu LF, Sun WJ, Zhang Q, Zhang W, Wang GC, Qin ZC, Yu LJ, Li HL, Zhang R (2020) Evaluation of CH4MODwetland and Terrestrial Ecosystem Model (TEM) used to estimate global CH4 emissions from natural wetlands. Geoscientific Model Development, 13, 3769-3788. |
| [36] | Li WH, Siddique MS, Liu MJ, Graham N, Yu WZ (2022) The migration and microbiological degradation of dissolved organic matter in riparian soils. Water Research, 224, 119080. |
| [37] | Li YS, Li YM, Ouyang ZY (2014) A research overview of methanogens. Environmental Science, 35, 2025-2030. (in Chinese with English abstract) |
| [李煜珊, 李耀明, 欧阳志云 (2014) 产甲烷微生物研究概况. 环境科学, 35, 2025-2030.] | |
| [38] | Liu YC, Whitman WB (2008) Metabolic, phylogenetic, and ecological diversity of the methanogenic Archaea. Annals of the New York Academy of Sciences, 1125, 171-189. |
| [39] | Lofton DD, Whalen SC, Hershey AE (2014) Effect of temperature on methane dynamics and evaluation of methane oxidation kinetics in shallow Arctic Alaskan lakes. Hydrobiologia, 721, 209-222. |
| [40] | Lu Q, Zhang SY, Du JQ, Liu Q, Dong CX, Zhao JD, Wang YF, Yao M (2023) Multi-group biodiversity distributions and drivers of metacommunity organization along a glacial-fluvial-limnic pathway on the Tibetan Plateau. Environmental Research, 220, 115236. |
| [41] | Lyu Z, Shao NN, Akinyemi T, Whitman WB (2018) Methanogenesis. Current Biology, 28, R727-R732. |
| [42] | Machado-Silva F, Weintraub MN, Ward ND, Doro KO, Regier PJ, Ehosioke S, Thomas SP, Peixoto RB, Sandoval L, Forbrich I, Kemner KM, O’Loughlin EJ, Stetten L, Spanbauer T, Bridgeman TB, O’Meara T, Rod KA, Patel K, McDowell NG, Megonigal JP, Rich RL, Bailey VL (2024) Short-term groundwater level fluctuations drive subsurface redox variability. Environmental Science & Technology, 58, 14687-14697. |
| [43] | Mansour I, Heppell CM, Ryo M, Rillig MC (2018) Application of the microbial community coalescence concept to riverine networks. Biological Reviews, 93, 1832-1845. |
| [44] |
Mayumi D, Mochimaru H, Tamaki H, Yamamoto K, Yoshioka H, Suzuki Y, Kamagata Y, Sakata S (2016) Methane production from coal by a single methanogen. Science, 354, 222-225.
PMID |
| [45] | Milner AM, Khamis K, Battin TJ, Brittain JE, Barrand NE, Füreder L, Cauvy-Fraunié S, Gíslason GM, Jacobsen D, Hannah DM, Hodson AJ, Hood E, Lencioni V, Ólafsson JS, Robinson CT, Tranter M, Brown LE (2017) Glacier shrinkage driving global changes in downstream systems. Proceedings of the National Academy of Sciences, USA, 114, 9770-9778. |
| [46] | Mitsch WJ, Gosselink JG (2000) The value of wetlands: Importance of scale and landscape setting. Ecological Economics, 35, 25-33. |
| [47] |
Ozuolmez D, Na H, Lever MA, Kjeldsen KU, Jørgensen BB, Plugge CM (2015) Methanogenic archaea and sulfate reducing bacteria co-cultured on acetate: Teamwork or coexistence? Frontiers in Microbiology, 6, 492.
DOI PMID |
| [48] |
Picazo F, Vilmi A, Aalto J, Soininen J, Casamayor EO, Liu YQ, Wu QL, Ren LJ, Zhou JZ, Shen J, Wang JJ (2020) Climate mediates continental scale patterns of stream microbial functional diversity. Microbiome, 8, 92.
DOI PMID |
| [49] |
Ruiz-González C, Niño-García JP, del Giorgio PA (2015) Terrestrial origin of bacterial communities in complex boreal freshwater networks. Ecology Letters, 18, 1198-1206.
DOI PMID |
| [50] | Samuel BS, Hansen EE, Manchester JK, Coutinho PM, Henrissat B, Fulton R, Latreille P, Kim K, Wilson RK, Gordon JI (2007) Genomic and metabolic adaptations of Methanobrevibacter smithii to the human gut. Proceedings of the National Academy of Sciences, USA, 104, 10643-10648. |
| [51] | Seppey CVW, Cabrol L, Thalasso F, Gandois L, Lavergne C, Martinez-Cruz K, Sepulveda-Jauregui A, Aguilar-Muñoz P, Astorga-España MS, Chamy R, Dellagnezze BM, Etchebehere C, Fochesatto GJ, Gerardo-Nieto O, Mansilla A, Murray A, Sweetlove M, Tananaev N, Teisserenc R, Tveit AT, Van de Putte A, Svenning MM, Barret M (2023) Biogeography of microbial communities in high-latitude ecosystems: Contrasting drivers for methanogens, methanotrophs and global prokaryotes. Environmental Microbiology, 25, 3364-3386. |
| [52] | Serrano-Silva N, Sarria-Guzmán Y, Dendooven L, Luna-Guido M (2014) Methanogenesis and methanotrophy in soil: A review. Pedosphere, 24, 291-307. |
| [53] | Shen WY, Ji Y, Jia ZJ, Huang Q, Zhu XL, Ma J, Wang SW, Liu XL, Zhang GB, Xu H (2024) Historical water regime determines the methanogenic pathway response to the current soil: Water ratio. Soil and Tillage Research, 239, 106032. |
| [54] |
Shenhav L, Thompson M, Joseph TA, Briscoe L, Furman O, Bogumil D, Mizrahi I, Pe’er I, Halperin E (2019) FEAST: Fast expectation-maximization for microbial source tracking. Nature Methods, 16, 627-632.
DOI PMID |
| [55] |
Sloan WT, Lunn M, Woodcock S, Head IM, Nee S, Curtis TP (2006) Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environmental Microbiology, 8, 732-740.
PMID |
| [56] | Svensson BH, Rosswall T (1984) In situ methane production from acid peat in plant communities with different moisture regimes in a subarctic mire. Oikos, 43, 341. |
| [57] | Tang Q, Xue XF, Wang H, Xing P (2018) New knowledge of methanogens and methanotrophs in lake ecosystems. Journal of Lake Sciences, 30, 597-610. (in Chinese with English abstract) |
| [唐千, 薛校风, 王惠, 邢鹏 (2018) 湖泊生态系统产甲烷与甲烷氧化微生物研究进展. 湖泊科学, 30, 597-610.] | |
| [58] |
Thauer RK, Kaster AK, Seedorf H, Buckel W, Hedderich R (2008) Methanogenic Archaea: Ecologically relevant differences in energy conservation. Nature Reviews Microbiology, 6, 579-591.
DOI PMID |
| [59] | Tian H, Du Y, Deng YM, Sun XL, Xu JW, Gan YQ, Wang YX (2024) Identification of methane cycling pathways in Quaternary alluvial-lacustrine aquifers using multiple isotope and microbial indicators. Water Research, 250, 121027. |
| [60] | Tian W, Wang HM, Xiang X, Loni PC, Qiu X, Wang RC, Huang XY, Tuovinen OH (2023) Water table level controls methanogenic and methanotrophic communities and methane emissions in a Sphagnum-dominated peatland. Microbiology Spectrum, 11, e0199223. |
| [61] | Tian W, Wang RC, Wang HM, Xiang X, Huang XY (2024) Hydrology drives spatiotemporal patterns of methane microbial communities and methane emissions in a sub-alpine peatland, Central China. Agricultural and Forest Meteorology, 353, 110050. |
| [62] |
Turetsky MR, Kotowska A, Bubier J, Dise NB, Crill P, Hornibrook ERC, Minkkinen K, Moore TR, Myers-Smith IH, Nykänen H, Olefeldt D, Rinne J, Saarnio S, Shurpali N, Tuittila ES, Waddington JM, White JR, Wickland KP, Wilmking M (2014) A synthesis of methane emissions from 71 northern, temperate, and subtropical wetlands. Global Change Biology, 20, 2183-2197.
DOI PMID |
| [63] | Vellend M (2001) Do commonly used indices of β-diversity measure species turnover? Journal of Vegetation Science, 12, 545-552. |
| [64] | Vellend M, Srivastava DS, Anderson KM, Brown CD, Jankowski JE, Kleynhans EJ, Kraft NJB, Letaw AD, MacDonald AAM, MacLean JE, Myers-Smith IH, Norris AR, Xue XX (2014) Assessing the relative importance of neutral stochasticity in ecological communities. Oikos, 123, 1420-1430. |
| [65] |
Volmer JG, Soo RM, Evans PN, Hoedt EC, Astorga Alsina AL, Woodcroft BJ, Tyson GW, Hugenholtz P, Morrison M (2023) Isolation and characterisation of novel Methanocorpusculum species indicates the genus is ancestrally host-associated. BMC Biology, 21, 59.
DOI PMID |
| [66] |
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Applied and Environmental Microbiology, 73, 5261-5267.
DOI PMID |
| [67] |
Wen X, Yang SZ, Horn F, Winkel M, Wagner D, Liebner S (2017) Global biogeographic analysis of methanogenic Archaea identifies community-shaping environmental factors of natural environments. Frontiers in Microbiology, 8, 1339.
DOI PMID |
| [68] |
Wik M, Varner RK, Anthony KW, MacIntyre S, Bastviken D (2016) Climate-sensitive northern lakes and ponds are critical components of methane release. Nature Geoscience, 9, 99-105.
DOI |
| [69] | Xie L, Du SY, Bu F (2018) Homoacetogen and its application in environmental biotechnology. Journal of Tongji University (Natural Science), 46, 67-73, 108. (in Chinese with English abstract) |
| [谢丽, 杜诗云, 卜凡 (2018) 同型产乙酸菌研究进展及其环境生物技术应用. 同济大学学报(自然科学版), 46, 67-73, 108.] | |
| [70] |
Xiong W, Ni P, Chen YY, Gao YC, Shan BQ, Zhan AB (2017) Zooplankton community structure along a pollution gradient at fine geographical scales in river ecosystems: The importance of species sorting over dispersal. Molecular Ecology, 26, 4351-4360.
DOI PMID |
| [71] |
Xue C, Li BK, Lei TY, Shan HY, Kong HZ (2022) Advances on the origin and evolution of biodiversity. Biodiversity Science, 30, 22460. (in Chinese with English abstract)
DOI |
|
[薛成, 李波卡, 雷天宇, 山红艳, 孔宏智 (2022) 生物多样性起源与进化研究进展. 生物多样性, 30, 22460.]
DOI |
|
| [72] | Yang S, Wan RR, Li B (2022) Hydrological connectivity research in Lake Taihu Basin: Status, progress and future challenges. Journal of Lake Sciences, 34, 1055-1074. (in Chinese with English abstract) |
| [杨素, 万荣荣, 李冰 (2022) 太湖流域水文连通性: 现状、研究进展与未来挑战. 湖泊科学, 34, 1055-1074.] | |
| [73] | Yang SZ, Liebner S, Alawi M, Ebenhöh O, Wagner D (2014) Taxonomic database and cut-off value for processing mcrA gene 454 pyrosequencing data by MOTHUR. Journal of Microbiological Methods, 103, 3-5. |
| [74] | Yang SZ, Liebner S, Winkel M, Alawi M, Horn F, Dörfer C, Ollivier J, He JS, Jin HJ, Kühn P, Schloter M, Scholten T, Wagner D (2017) In-depth analysis of core methanogenic communities from high elevation permafrost-affected wetlands. Soil Biology and Biochemistry, 111, 66-77. |
| [75] | Yang XS, Wang S, He Q, Wang ZJ, Zhang ZJ, Jiang CY, Ma LP, Liu XW, Hu BL, Li YM, Deng Y (2021) Microorganisms in the typical anaerobic digestion system of organic solid wastes: A review. Chinese Journal of Biotechnology, 37, 3425-3438. (in Chinese with English abstract) |
| [杨兴盛, 王尚, 何晴, 王朱珺, 张照婧, 姜成英, 马黎萍, 刘贤伟, 胡宝兰, 李咏梅, 邓晔 (2021) 典型有机固废厌氧消化微生物研究现状与发展方向. 生物工程学报, 37, 3425-3438.] | |
| [76] | Yi Y, Zhou Z, Huang Y, Cheng L (2023) Methanogen research in China: Current status and prospective. Acta Microbiologica Sinica, 63, 1796-1814. (in Chinese with English abstract) |
| [易悦, 周卓, 黄艳, 承磊 (2023) 我国产甲烷古菌研究进展与展望. 微生物学报, 63, 1796-1814.] | |
| [77] | Yuan J, Yuan YK, Zhu YH, Cao LK (2018) Effects of different fertilizers on methane emissions and methanogenic community structures in paddy rhizosphere soil. Science of the Total Environment, 627, 770-781. |
| [78] | Zhang B, Wu YH, Zhu LP, Wang JB, Li JS, Chen DM (2011) Estimation and trend detection of water storage at Nam Co Lake, central Tibetan Plateau. Journal of Hydrology, 405, 161-170. |
| [79] | Zhang HJ, Xu HM, Wang SR, Qin MY, Zhao DY, Wu QL, Zeng J (2023) Habitats modulate influencing factors shaping the spatial distribution of bacterial communities along a Tibetan Plateau riverine wetland. Science of the Total Environment, 860, 160418. |
| [80] | Zhang WT, Kang XM, Kang EZ, Audet J, Davidson TA, Zhang XD, Yan L, Li Y, Yan ZQ, Zhang KR, Wang JZ, Hu ZY (2022) Soil water content, carbon, and nitrogen determine the abundances of methanogens, methanotrophs, and methane emission in the Zoige alpine wetland. Journal of Soils and Sediments, 22, 470-481. |
| [81] | Zhang ZS, Yu XJ, Song XL, Xue ZS, Wu HT (2019) Impacts of hydrological connectivity on key ecological processes and functions in wetlands: A general review. Wetland Science, 17, 1-8. (in Chinese with English abstract) |
| [张仲胜, 于小娟, 宋晓林, 薛振山, 武海涛 (2019) 水文连通对湿地生态系统关键过程及功能影响研究进展. 湿地科学, 17, 1-8.] | |
| [82] | Zhou YX, Chen KY, Muneer MA, Li CC, Shi HL, Tang Y, Zhang J, Ji BM (2022) Soil moisture and pH differentially drive arbuscular mycorrhizal fungal composition in the riparian zone along an alpine river of Nam Co watershed. Frontiers in Microbiology, 13, 994918. |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn