



生物多样性 ›› 2020, Vol. 28 ›› Issue (8): 1018-1025. DOI: 10.17520/biods.2019366 cstr: 32101.14.biods.2019366
刘丽平1, 宋瑞凤1, 张馥1,3, 张秀香1, 彭桂香2,*( ), 谭志远1,*(
), 谭志远1,*( )
)
收稿日期:2019-11-12
接受日期:2020-03-03
出版日期:2020-08-20
发布日期:2020-06-12
通讯作者:
彭桂香,谭志远
作者简介:zytan@scau.edu.cn基金资助:
Liping Liu1, Ruifeng Song1, Fu Zhang1,3, Xiuxiang Zhang1, Guixiang Peng2,*( ), Zhiyuan Tan1,*(
), Zhiyuan Tan1,*( )
)
Received:2019-11-12
Accepted:2020-03-03
Online:2020-08-20
Published:2020-06-12
Contact:
Guixiang Peng,Zhiyuan Tan
摘要:
高秆野生稻(Oryza alta)是一种重要的种质资源, 其组织内也蕴藏着非常宝贵的功能微生物资源。本实验采用无氮培养基, 从高秆野生稻中分离到43株内生固氮菌, 结合乙炔还原法测定其固氮酶活性。经固氮酶基因(nifH)的PCR扩增检测, 43株内生固氮菌代表菌株均能扩增出固氮酶基因片段。利用IS-PCR DNA指纹图谱和SDS-PAGE全细胞蛋白电泳图谱将获得的菌株聚类为6个类群(I、II、III、IV、V、VI)。对各个类群的代表菌株(ZF3, ZF8, ZF13, ZF15, ZF24, ZF43)进行16S rRNA基因序列测定, 结果表明, 类群I属于土生拉乌尔菌(Raoultella terrigena), 类群II属于类肺炎克雷伯氏菌类肺炎亚种(Klebsiella quasipnmoniae subsp. quasipneumoniae), 类群III属于越南伯克氏菌(Burkholderia vietnamiensis), 类群IV的菌株代表肠秆菌属的一个类群(Enterobacter sp.), 类群V的菌株属于门多萨假单胞菌(Pseudomonas mendocina), 类群VI归属于固氮植物菌(Phytobacter diazotrophicus)。Biolog聚类结果与IS-PCR指纹图谱类型及SDS-PAGE全细胞蛋白聚类结果一致。Biolog板测定结果显示, 来自不同类群的代表菌株对碳源的利用差异显著, 说明野生稻内多样的内生固氮菌从环境中获取碳源和氮素的适应能力较强。
刘丽平, 宋瑞凤, 张馥, 张秀香, 彭桂香, 谭志远 (2020) 高秆野生稻内生固氮细菌多样性. 生物多样性, 28, 1018-1025. DOI: 10.17520/biods.2019366.
Liping Liu, Ruifeng Song, Fu Zhang, Xiuxiang Zhang, Guixiang Peng, Zhiyuan Tan (2020) Diversity of endophytic diazotrophs isolated from Oryza alta. Biodiversity Science, 28, 1018-1025. DOI: 10.17520/biods.2019366.
| 菌株编号 Strain no. | 部位 Organ | 固氮酶活性 Nitrogenase activity (nmol C2H2/mL·h) | 菌株编号 Strain no. | 部位 Organ | 固氮酶活性 Nitrogenase activity (nmol C2H2/mL·h) |
|---|---|---|---|---|---|
| ZF1 | 根 Root | 10.82 ± 0.15 | ZF23 | 叶 Leave | 72.63 ± 0.93 |
| ZF2 | 叶 Leave | 26.19 ± 0.19 | ZF24 | 叶 Leave | 57.67 ± 0.45 |
| ZF3 | 茎 Stem | 15.76 ± 0.31 | ZF25 | 茎 Stem | 59.60 ± 0.41 |
| ZF4 | 茎 Stem | 10.72 ± 0.24 | ZF26 | 茎 Stem | 13.18 ± 0.18 |
| ZF5 | 茎 Stem | 15.39 ± 0.28 | ZF27 | 茎 Stem | 184.15 ± 1.05 |
| ZF6 | 茎 Stem | 20.22 ± 0.39 | ZF28 | 叶 Leave | 67.17 ± 0.79 |
| ZF7 | 叶 Leave | 76.00 ± 0.45 | ZF29 | 根 Root | 10.91 ± 0.22 |
| ZF8 | 根 Root | 254.31 ± 4.51 | ZF30 | 根 Root | 5.44 ± 0.15 |
| ZF9 | 根 Root | 26.09 ± 0.29 | ZF31 | 茎 Stem | 257.42 ± 1.57 |
| ZF10 | 茎 Stem | 506.46 ± 3.63 | ZF32 | 叶 Leave | 29.41 ± 0.33 |
| ZF11 | 茎 Stem | 1.23 ± 0.11 | ZF33 | 茎 Stem | 1.82 ± 0.17 |
| ZF12 | 根 Root | 69.44 ± 0.65 | ZF34 | 茎 Stem | 43.62 ± 0.43 |
| ZF13 | 茎 Stem | 17.20 ± 0.16 | ZF35 | 茎 Stem | 87.18 ± 0.55 |
| ZF14 | 叶 Leave | 41.82 ± 0.39 | ZF36 | 茎 Stem | 132.52 ± 0.66 |
| ZF15 | 茎 Stem | 59.84 ± 0.48 | ZF37 | 根 Root | 90.59 ± 0.48 |
| ZF16 | 根 Root | 94.03 ± 0.57 | ZF38 | 茎 Stem | 33.36 ± 0.34 |
| ZF17 | 根 Root | 107.85 ± 1.11 | ZF39 | 茎 Stem | 9.67 ± 0.19 |
| ZF18 | 茎 Stem | 33.02 ± 0.43 | ZF40 | 根 Root | 98.96 ± 0.51 |
| ZF19 | 茎 Stem | 11.74 ± 0.21 | ZF41 | 叶 Leave | 35.50 ± 0.43 |
| ZF20 | 茎 Stem | 108.38 ± 0.67 | ZF42 | 茎 Stem | 38.05 ± 0.42 |
| ZF21 | 根 Root | 64.74 ± 0.71 | ZF43 | 茎 Stem | 80.49 ± 0.59 |
| ZF22 | 茎 Stem | 20.50 ± 0.23 |
表1 高秆野生稻内生固氮菌来源及固氮酶活性测定(固氮酶活性为3次测定的平均值)
Table 1 Origin of isolates and nitrogenase activity from Oryza alta L.The activity of nitrogenase was the average value of three determinations.
| 菌株编号 Strain no. | 部位 Organ | 固氮酶活性 Nitrogenase activity (nmol C2H2/mL·h) | 菌株编号 Strain no. | 部位 Organ | 固氮酶活性 Nitrogenase activity (nmol C2H2/mL·h) |
|---|---|---|---|---|---|
| ZF1 | 根 Root | 10.82 ± 0.15 | ZF23 | 叶 Leave | 72.63 ± 0.93 |
| ZF2 | 叶 Leave | 26.19 ± 0.19 | ZF24 | 叶 Leave | 57.67 ± 0.45 |
| ZF3 | 茎 Stem | 15.76 ± 0.31 | ZF25 | 茎 Stem | 59.60 ± 0.41 |
| ZF4 | 茎 Stem | 10.72 ± 0.24 | ZF26 | 茎 Stem | 13.18 ± 0.18 |
| ZF5 | 茎 Stem | 15.39 ± 0.28 | ZF27 | 茎 Stem | 184.15 ± 1.05 |
| ZF6 | 茎 Stem | 20.22 ± 0.39 | ZF28 | 叶 Leave | 67.17 ± 0.79 |
| ZF7 | 叶 Leave | 76.00 ± 0.45 | ZF29 | 根 Root | 10.91 ± 0.22 |
| ZF8 | 根 Root | 254.31 ± 4.51 | ZF30 | 根 Root | 5.44 ± 0.15 |
| ZF9 | 根 Root | 26.09 ± 0.29 | ZF31 | 茎 Stem | 257.42 ± 1.57 |
| ZF10 | 茎 Stem | 506.46 ± 3.63 | ZF32 | 叶 Leave | 29.41 ± 0.33 |
| ZF11 | 茎 Stem | 1.23 ± 0.11 | ZF33 | 茎 Stem | 1.82 ± 0.17 |
| ZF12 | 根 Root | 69.44 ± 0.65 | ZF34 | 茎 Stem | 43.62 ± 0.43 |
| ZF13 | 茎 Stem | 17.20 ± 0.16 | ZF35 | 茎 Stem | 87.18 ± 0.55 |
| ZF14 | 叶 Leave | 41.82 ± 0.39 | ZF36 | 茎 Stem | 132.52 ± 0.66 |
| ZF15 | 茎 Stem | 59.84 ± 0.48 | ZF37 | 根 Root | 90.59 ± 0.48 |
| ZF16 | 根 Root | 94.03 ± 0.57 | ZF38 | 茎 Stem | 33.36 ± 0.34 |
| ZF17 | 根 Root | 107.85 ± 1.11 | ZF39 | 茎 Stem | 9.67 ± 0.19 |
| ZF18 | 茎 Stem | 33.02 ± 0.43 | ZF40 | 根 Root | 98.96 ± 0.51 |
| ZF19 | 茎 Stem | 11.74 ± 0.21 | ZF41 | 叶 Leave | 35.50 ± 0.43 |
| ZF20 | 茎 Stem | 108.38 ± 0.67 | ZF42 | 茎 Stem | 38.05 ± 0.42 |
| ZF21 | 根 Root | 64.74 ± 0.71 | ZF43 | 茎 Stem | 80.49 ± 0.59 |
| ZF22 | 茎 Stem | 20.50 ± 0.23 |
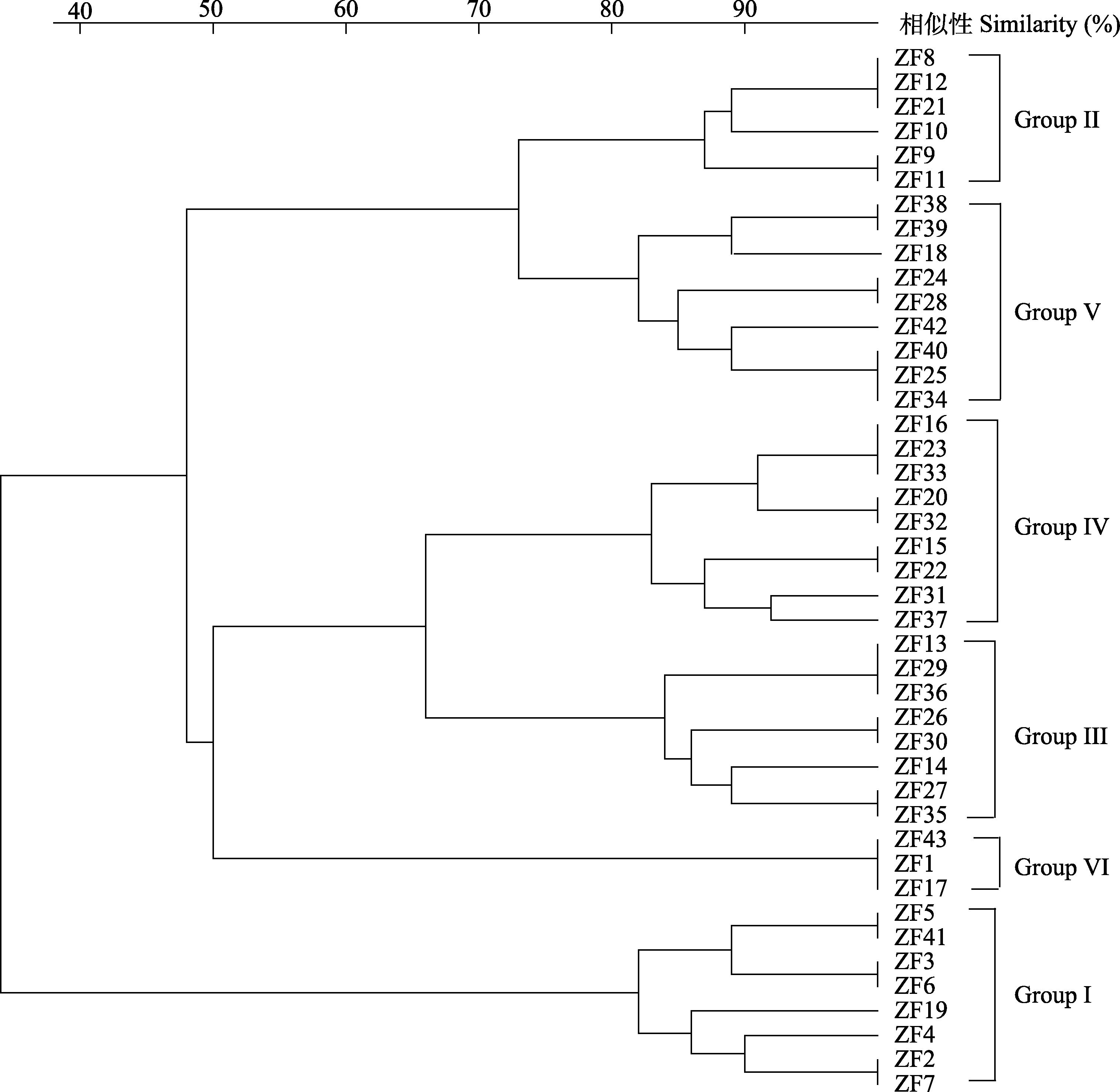
图1 高秆野生稻43株内生固氮菌(菌株编号及特征见表1)的IS-PCR指纹图谱聚类(UPGMA)
Fig. 1 Dendrogram obtained by UPGMA method showing the similarity of IS-PCR patterns of 43 endophytic diazotrophs isolated from Oryza alta . The strain numbers and characters of isolates see Table 1.
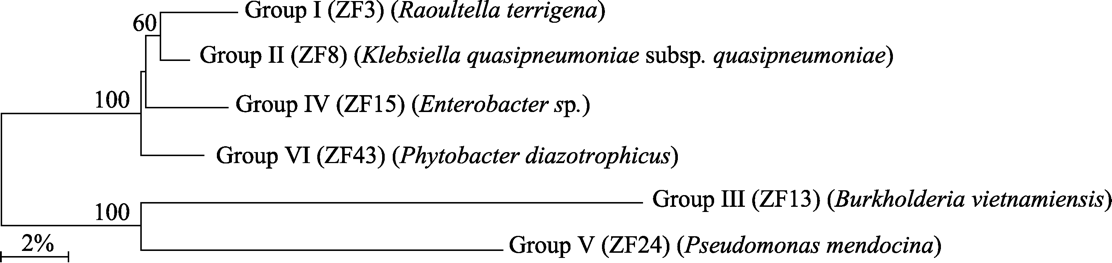
图2 基于16S rRNA基因序列的高秆野生稻各类群代表菌株的Neighbor-joining树(分支数字代表自展支持率, 标尺代表核苷酸碱基差异为2%, 随机抽样计算次数为1,000)
Fig. 2 Neighbor-joining tree based on the 16S rRNA gene sequences of representative strains of each group isolated from Oryza alta. Branch numbers represent the support rate of self-development. Scales represent the difference of nucleotide bases, which is 2%. The number of random sampling calculations is 1,000.
| 类群 Groups | 代表菌株 Representative strain | 最相似菌株名称(GenBank登录号) Closely related strain (GenBank accession number) | 相似性 Similarity (%) |
|---|---|---|---|
| I | ZF3 | 土生乌拉尔菌 Raoultella terrigena ATCC33257T (NR_114503) | 99.79 |
| II | ZF8 | 类肺炎克雷伯氏菌肺炎亚种 Klebsiella quasipneumoniae subsp. quasipneumoniae 01A030T (HG933296) | 99.86 |
| III | ZF13 | 越南伯克霍尔德氏菌 Burkholderia vietnamiensis LMG10929T (AF097534) | 99.93 |
| IV | ZF15 | 肠杆菌 Enterobacter sp. R4-368 (CP005991_s) | 99.59 |
| V | ZF24 | 门多萨假单胞菌 Pseudomonas mendocina NBRC14162T (BBQC01000018) | 99.03 |
| VI | ZF43 | 固氮植物菌 Phytobacter diazotrophicus LS8T (DQ821583.2) | 99.37 |
表2 高秆野生稻各类群代表菌株16S rDNA序列相似性分析结果
Table 2 Similarity of 16S rDNA sequences of representative strain of each group isolated from Oryza alta
| 类群 Groups | 代表菌株 Representative strain | 最相似菌株名称(GenBank登录号) Closely related strain (GenBank accession number) | 相似性 Similarity (%) |
|---|---|---|---|
| I | ZF3 | 土生乌拉尔菌 Raoultella terrigena ATCC33257T (NR_114503) | 99.79 |
| II | ZF8 | 类肺炎克雷伯氏菌肺炎亚种 Klebsiella quasipneumoniae subsp. quasipneumoniae 01A030T (HG933296) | 99.86 |
| III | ZF13 | 越南伯克霍尔德氏菌 Burkholderia vietnamiensis LMG10929T (AF097534) | 99.93 |
| IV | ZF15 | 肠杆菌 Enterobacter sp. R4-368 (CP005991_s) | 99.59 |
| V | ZF24 | 门多萨假单胞菌 Pseudomonas mendocina NBRC14162T (BBQC01000018) | 99.03 |
| VI | ZF43 | 固氮植物菌 Phytobacter diazotrophicus LS8T (DQ821583.2) | 99.37 |
| [1] | Baldani VLD, Alvarez MA, Baldani JI (1986) Establishment of inoculated Azospirillum spp. in the rhizosphere and in roots of field grown wheat and sorghum. Plant & Soil, 90, 35-46. |
| [2] | Belviso S, Giordano M, Ambrosoli R, Minati JL, Bertolino M, Zeppa G (2014) Assessment of lactic acid bacteria sensitivity to terpenoids with the Biolog methodology. Dairy Science & Technology, 94, 195-203. |
| [3] | Chen K, Li JS, Yang HT, Wei YL, Huang YJ, Zhou HZ (2008) Biocontrol and yield-increasing effects of Burkholderia vietnamiensis recombinant strain B418-37 in greenhouse. Shandong Agricultural Sciences, ( 7), 58-60. (in Chinese with English abstract) |
| [ 陈凯, 李纪顺, 杨合同, 魏艳丽, 黄玉杰, 周红姿 (2008) 越南伯克霍尔德氏工程菌株B418-37的温室防病增产效果. 山东农业科学, ( 7), 58-60.] | |
| [4] | Döbereiner J, Day JM (1976) Associative symbioses in tropical Grasses: Characterization of microorganisms and dinitrogen-fixing sites. In: Proceedings of the 1st International Symposium on Nitrogen Fixation (eds Newton WE, Nyman CJ), pp. 518-538. Washington State University Press, Pullman. |
| [5] |
Drancourt M, Bollet C, Carta A, Rousselier P (2001) Phylogenetic analyses of Kebsiella species delineate Klebsiella and Raoultella gen. nov., with description of Raoultella ornithinolytica comb. nov., Raoultella terrigena comb. nov. and Raoultella planticola comb. nov. International Journal of Systematic and Evolutionary Microbiology, 51, 925-932.
URL PMID |
| [6] | Gao J, Jiang HS, Wang ZY (2013) The degradation of PHBV by Pseudomonas mendocina DS04-T. Journal of Liaoning University of Petroleum & Chemical Technology, 33(3), 4-7. (in Chinese with English abstract) |
| [ 高佳, 姜虎生, 王战勇 (2013) 门多萨假单胞菌DS04-T对PHBV降解性能研究. 辽宁石油化工大学学报, 333, 4-7.] | |
| [7] |
Gryta A, Frąc M, Oszust K (2014) The application of the Biolog ecoplate approach in ecotoxicological evaluation of dairy sewage sludge. Applied Biochemistry & Biotechnology, 174, 1434-1443.
DOI URL PMID |
| [8] | Hu WZ, Tan ZW, Wang Y, Xu XW, Tan ZY (2016) Isolation, identification and phylogenetic analysis of endophytic diazotrophs from Oryza officinalis grown in Tengxian County. Biotechnology Bulletin, 32(6), 111-119. (in Chinese with English abstract) |
| [ 胡文哲, 谭泽文, 王勇, 徐羡微, 谭志远 (2016) 藤县药用野生稻内生固氮菌分离鉴定及系统发育分析. 生物技术通报, 326, 111-119.] | |
| [9] | Liu LF, Cun HC, He PF, Di YN, Wu YX, He LL, Li FS, He YQ (2019) Isolation, identification and multiple function analyses of sugarcane endophytes. Chinese Journal of Tropical Crops, 40, 1144-1152. (in Chinese with English abstract) |
| [ 刘鲁峰, 寸海春, 何鹏飞, 狄义宁, 吴毅歆, 何丽莲, 李富生, 何月秋 (2019) 甘蔗内生菌分离鉴定及功能多样性研究. 热带作物学报, 40, 1144-1152.] | |
| [10] | Liu LH, Jiang HM, Wang PX, Tang XY, Peng GX, Tan ZY (2017) Research progress on diversity of endophytic diazotrophs in wild rices. Current Biotechnology, 7, 567-579. (in Chinese with English abstract) |
| [ 刘丽辉, 蒋慧敏, 王佩旋, 唐小钰, 彭桂香, 谭志远 (2017) 野生稻内生固氮菌多样性研究进展. 生物技术进展, 7, 567-579.] | |
| [11] | Malik K, Rasul G, Hassan U, Mehnaz S, Ashraf M (1993) Role of N2-fixing and growth hormone producing bacteria in improving growth of wheat and rice. In: The Sixth International Symposium on Nitrogen Fixation with Non-legumes (eds Hegazi NA, Fayez M, Monib M), The American University in Cairo Press, Cairo. |
| [12] | Mashiane AR, Adeleke RA, Bezuidenhout CC, Chirima GJ (2018) Community composition and functions of endophytic bacteria of Bt maize. South African Journal of Science, 114, 88-97. |
| [13] | Palus JA, Borneman J, Ludden PW, Triplett EW (1996) A diazotrophic bacterial endophyte isolated from stems of Zea mays L. and Zea luxurians Iltis and Doebley. Plant Soil, 186, 135-142. |
| [14] |
Paungfoo-Lonhienne C, Lonhienne TG, Yeoh YK, Webb RI, Lakshmanan P, Chan CX, Lim PE, Ragan MA, Schmidt S, Hugenholtz P (2014) A new species of Burkholderia isolated from sugarcane roots promotes plant growth. Microbial Biotechnology, 7, 142-154.
DOI URL PMID |
| [15] | Ruggiero NA, Chang M, Brown S (2016) Pseudomonas mendocina bacteremia: A case study and review of literature. Infectious Diseases in Clinical Practice, 24, 314-317. |
| [16] | Shabanamol S, Divya K, George TK, Rishad KS, Sreekumar TS, Jisha MS (2018) Characterization and in plant nitrogen fixation of plant growth promoting endophytic diazotrophic Lysinibacillus sphaericus isolated from rice (Oryza sativa). Physiological and Molecular Plant Pathology, 102, 46-54. |
| [17] | Shi GY, Zeng Q, Nong ZM, Ye XL, Cen ZL, Li YR, Hu CJ (2019) Identification of an endophytic nitrogen-fixing bacterium NN08200 from sugarcane and its growth promotion of sugarcane. Microbiology China, 46, 1336-1345. (in Chinese with English abstract) |
| [ 史国英, 曾泉, 农泽梅, 叶雪莲, 岑贞陆, 李杨瑞, 胡春锦 (2019) 一株高效甘蔗内生固氮菌NN08200的鉴定及其对甘蔗的促生长作用. 微生物学通报, 46, 1336-1345.] | |
| [18] | Tan ZY, Chen WX (1998) SDS-PAGE of whole cell protein of new rhizobial groups and 16S rDNA sequencing of their repentatives. Chinese Journal of Applied and Environmental Biology , 4, 65-69. (in Chinese with English abstract) |
| [ 谭志远, 陈文新 (1998) 根瘤菌新类群的全细胞蛋白电泳及16S rDNA全序列分析. 应用与环境生物学报, 4, 65-69.] | |
| [19] |
Tan ZY, Hurek T, Vinuesa P, Mueller P, Ladha JK, Reinhold- Hurek B (2001) Specific detection of Bradyrhizobium and Rhizobium strains colonizing rice (Oryza sativa) roots by 16S-23S ribosomal DNA intergenic spacer-targeted PCR. Applied and Environmental Microbiology, 67, 3655-3664.
DOI URL PMID |
| [20] | Tang SY, Hara S, Melling L, Kah-Joo G, Hashidoko Y (2010) Burkholderia vietnamiensis isolated from root tissues of nipa palm (Nypa fruticans) in Sarawak, Malaysia, proved to be its major endophytic nitrogen-fixing bacterium. Journal of the Agricultural Chemical Society of Japan, 74, 1972-1975. |
| [21] | Tian H, Zhang DG, Yao T, Xi LQ (2005) Isolation and characteristics of nitrogen fixation bacteria in rhizosphere of turfgrass. Grassland of China, 27(5), 49-54. (in Chinese with English abstract) |
| [ 田宏, 张德罡, 姚拓, 席琳乔 (2005) 禾本科草坪草固氮菌株筛选及部分特性初步研究. 中国草地, 275, 49-54.] | |
| [22] | Trân VV, Berge O, Balandreau J, Kê SN, Heulin T (1996) Isolation and nitrogenase activity of Burkholderia vietnamiensis, a nitrogen-fixing bacterium associated with rice (Oryza sativa L) on a sulphate acid soil of Vietnam. Agronomie, 16, 479-481. |
| [23] |
Verma SC, Ladha JK, Tripathi AK (2001) Evaluation of plant growth promoting and colonization ability of endophytic diazotrophs from deep water rice. Journal of Biotechnology, 91, 127-141.
DOI URL PMID |
| [24] | Wang JR, Chen LZ, Luo CC, Zhong KX, Nie JM, Li YY (2013) Cloning of the lipase gene of Pseudomonas mendocina P10 and its expression in Pichia pastoris. Science and Technology of Food Industry, 34(12), 169-172. (in Chinese with English abstract) |
| [ 王建荣, 陈丽芝, 罗长财, 钟开新, 聂金梅, 李阳源 (2013) 门多萨假单胞菌Pseudomonas mendocina P10脂肪酶基因的克隆及其在酵母中的表达. 食品工业科技, 3412, 169-172.] | |
| [25] | Wang YL, Chen K, Li Z, Wu YZ, Guo K, , Li JS, Yang HT (2014) Isolation and identification of nematicidal active substance from Burkholderia vietnamiensis B418. Plant Protection, 40(4), 65-69. (in Chinese with English abstract) |
| [ 王贻莲, 陈凯, 李哲, 吴远征, 郭凯, 李纪顺, 杨合同 (2014) 越南伯克霍尔德氏菌B418杀线虫活性产物的分离鉴定. 植物保护, 404, 65-69. | |
| [26] | Wei GH, Zhu ME (1999) Applications of molecular biological methods on taxonomy of rhizobia. Journal of Northwest Sci- Tech University of Agriculture and Forestry (Natural Science Edition), 27(2), 3-5. (in Chinese with English abstract) |
| [ 韦革宏, 朱铭莪 (1999) 分子生物学新方法在根瘤菌分类中的应用. 西北农业大学学报, 272, 3-5.] | |
| [27] |
Zhang GX, Peng GX, Wang ET, Yan H, Yuan Q H, Zhang W, Lou X, Wu H, Tan ZY (2008) Diverse endophytic nitrogen-fixing bacteria isolated from wild rice Oryza rufipogon and description of Phytobacter diazotrophicus gen. nov. sp. Nov. Archives of Microbiology, 189, 431-439
DOI URL PMID |
| [28] | Zhao XW, Peng GX, Zhang ZY, Qiu YX, Zhang ZM, Zhang GQ, Tan ZY (2010) A novel grouping method: ddT clustering assessment of the diversity of associated nitrogen-fixing bacteria isolated from Vetiveria zizanioides. Chinese Science Bulletin, 55, 562-571. (in Chinese with English abstract) |
| [ 赵现伟, 彭桂香, 张志英, 邱永雄, 张泽民, 张桂权, 谭志远 (2010) 细菌聚类新方法: ddT聚类技术分析香根草联合固氮菌多样性. 科学通报, 55, 562-571.] |
| [1] | 吴晓晴 张美惠 葛苏婷 李漫淑 宋坤 沈国春 达良俊 张健. 上海近自然林重建过程中木本植物物种多样性与地上生物量的时空动态——以闵行区生态岛为例[J]. 生物多样性, 2025, 33(5): 24444-. |
| [2] | 干靓 刘巷序 鲁雪茗 岳星. 全球生物多样性热点地区大城市的保护政策与优化方向[J]. 生物多样性, 2025, 33(5): 24529-. |
| [3] | 曾子轩 杨锐 黄越 陈路遥. 清华大学校园鸟类多样性特征与环境关联[J]. 生物多样性, 2025, 33(5): 24373-. |
| [4] | 周昊, 王茗毅, 张楚格, 肖治术, 欧阳芳. 昆虫旅馆在独栖蜂多样性保护中的现状与挑战[J]. 生物多样性, 2025, 33(5): 24472-. |
| [5] | 臧明月, 刘立, 马月, 徐徐, 胡飞龙, 卢晓强, 李佳琦, 于赐刚, 刘燕. 《昆明-蒙特利尔全球生物多样性框架》下的中国城市生物多样性保护[J]. 生物多样性, 2025, 33(5): 24482-. |
| [6] | 祝晓雨, 王晨灏, 王忠君, 张玉钧. 城市绿地生物多样性研究进展与展望[J]. 生物多样性, 2025, 33(5): 25027-. |
| [7] | 袁琳, 王思琦, 侯静轩. 大都市地区的自然留野:趋势与展望[J]. 生物多样性, 2025, 33(5): 24481-. |
| [8] | 胡敏, 李彬彬, Coraline Goron. 只绿是不够的: 一个生物多样性友好的城市公园管理框架[J]. 生物多样性, 2025, 33(5): 24483-. |
| [9] | 王欣, 鲍风宇. 基于鸟类多样性提升的南滇池国家湿地公园生态修复效果分析[J]. 生物多样性, 2025, 33(5): 24531-. |
| [10] | 明玥, 郝培尧, 谭铃千, 郑曦. 基于城市绿色高质量发展理念的中国城市生物多样性保护与提升研究[J]. 生物多样性, 2025, 33(5): 24524-. |
| [11] | 徐欢, 辛凤飞, 施宏亮, 袁琳, 薄顺奇, 赵欣怡, 邓帅涛, 潘婷婷, 余婧, 孙赛赛, 薛程. 生态修复技术集成应用对长江口北支生境与鸟类多样性提升效果评估[J]. 生物多样性, 2025, 33(5): 24478-. |
| [12] | 谢淦, 宣晶, 付其迪, 魏泽, 薛凯, 雒海瑞, 高吉喜, 李敏. 草地植物多样性无人机调查的物种智能识别模型构建[J]. 生物多样性, 2025, 33(4): 24236-. |
| [13] | 王太, 宋福俊, 张永胜, 娄忠玉, 张艳萍, 杜岩岩. 河西走廊内陆河水系鱼类多样性及资源现状[J]. 生物多样性, 2025, 33(4): 24387-. |
| [14] | 褚晓琳, 张全国. 演化速率假说的实验验证研究进展[J]. 生物多样性, 2025, 33(4): 25019-. |
| [15] | 张浩斌, 肖路, 刘艳杰. 夜间灯光对外来入侵植物和本地植物群落多样性和生长的影响[J]. 生物多样性, 2025, 33(4): 24553-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn