



生物多样性 ›› 2014, Vol. 22 ›› Issue (5): 649-657. DOI: 10.3724/SP.J.1003.2014.13257 cstr: 32101.14.SP.J.1003.2014.13257
所属专题: 海洋生物多样性
陈雪1,3, 张武昌1,*( ), 吴强2, 栾青杉2, 肖天1
), 吴强2, 栾青杉2, 肖天1
收稿日期:2013-12-11
接受日期:2014-04-17
出版日期:2014-09-20
发布日期:2014-10-09
通讯作者:
张武昌
基金资助:
Xue Chen1,3, Wuchang Zhang1,*( ), Qiang Wu2, Qingshan Luan2, Tian Xiao1
), Qiang Wu2, Qingshan Luan2, Tian Xiao1
Received:2013-12-11
Accepted:2014-04-17
Online:2014-09-20
Published:2014-10-09
Contact:
Zhang Wuchang
摘要:
为揭示莱州湾砂壳纤毛虫群落季节变化规律, 在莱州湾设置8个站位, 于2011年5-11月及2012年3-4月进行了9个航次的调查, 用浅海III型浮游生物网由底至表垂直拖网采集砂壳纤毛虫。结果表明, 莱州湾3-11月砂壳纤毛虫物种丰富度的变化范围为5-19, 周年变化呈现一峰两谷的趋势。丰度的范围为0-318 ind./L, 丰度较大(> 50 ind./L)的种类有运动类铃虫(Codonellopsis mobilis)和清兰拟铃虫(Tintinnopsis chinglanensis)。各月平均丰度随时间的变化趋势为双峰型, 最大值出现在7月(63 ind./L), 次峰值出现在5月(48 ind./L), 最小值出现在3月(2 ind./L)。黏着壳种类在3-11月均有出现, 透明壳种类仅在温度较高(> 15°C)的6-9月出现。各月的优势种数目为1种(5月)到8种(8月), 其中运动类铃虫在所有月中都是优势种, 对砂壳纤毛虫丰度周年的变化规律产生较大影响。使用各月所有种类的平均丰度对各月砂壳纤毛虫群落进行聚类分析, 得到两个群落(相似度30%): 群落I(7-9月)和群落II(3-6月、10-11月), 说明砂壳纤毛虫群落发生了明显的季节变化。砂壳纤毛虫的物种丰富度、丰度与环境因子(温度、盐度)均没有明显的相关性。
陈雪, 张武昌, 吴强, 栾青杉, 肖天 (2014) 莱州湾大型砂壳纤毛虫群落季节变化. 生物多样性, 22, 649-657. DOI: 10.3724/SP.J.1003.2014.13257.
Xue Chen, Wuchang Zhang, Qiang Wu, Qingshan Luan, Tian Xiao (2014) Seasonal change of the community of large-sized tintinnids (Ciliophora, Tintinnida) in Laizhou Bay. Biodiversity Science, 22, 649-657. DOI: 10.3724/SP.J.1003.2014.13257.
| 中文种名 Chinese name | 拉丁文种名 Latin name | Amax | Mmax |
|---|---|---|---|
| 透明壳种类 Hyaline species | |||
| 尖底类瓮虫 | Amphorellopsis acuta | 2.81 | 8 |
| 卢氏真铃虫 | Eutintinnus lusus-undae | 2.53 | 7 |
| 巴拿马网纹虫 | Favella panamensis | 4.21 | 6 |
| 黏着壳种类 Agglutinated species | |||
| 鲁西塔尼亚类铃虫 | Codonellopsis lusitanica | 1.04 | 8 |
| 运动类铃虫 | C. mobilis | 316.72 | 5 |
| 诺氏薄铃虫 | Leprotintinnus nordqvisti | 0.42 | 7 |
| 简单薄铃虫 | L. simplex | 7.67 | 7 |
| 白领细壳虫 | Stenosemella nivalis | 16.93 | 7 |
| 巴西拟铃虫 | Tintinnopsis brasiliensis | 1.04 | 3 |
| 布氏拟铃虫 | T. butschlii | 1.18 | 7 |
| 清兰拟铃虫 | T. chinglanensis | 65.85 | 7 |
| 有角拟铃虫 | T. corniger | 3.87 | 7 |
| 指状拟铃虫 | T. digita | 9.44 | 8 |
| 直颈拟铃虫 | T. directa | 0.53 | 7 |
| 半旋拟铃虫 | T. hemispiralis | 3.85 | 11 |
| 日本拟铃虫 | T. japonica | 14.21 | 4 |
| 卡拉直克拟铃虫 | T. karajacensis | 0.20 | 3 |
| 罗氏拟铃虫 | T. lohmanni | 6.15 | 7 |
| 梅氏拟铃虫 | T. mayeri | 0.11 | 4 |
| 根状拟铃虫 | T. radix | 29.14 | 7 |
| 圆锥拟铃虫 | T. rapa | 0.59 | 3 |
| 斯氏拟铃虫 | T. schotti | 12.43 | 9 |
| 妥肯丁拟铃虫 | T. tocantinensis | 13.38 | 8 |
| 未定种1 | Tintinnopsis sp.1 | 4.84 | 5 |
| 未定种2 | Tintinnopsis sp.2 | 0.39 | 6 |
| 未定种3 | Tintinnopsis sp.3 | 0.80 | 8 |
表1 莱州湾3-11月记录的砂壳纤毛虫种类
Table 1 Species list of tintinnids in Laizhou Bay from March to November
| 中文种名 Chinese name | 拉丁文种名 Latin name | Amax | Mmax |
|---|---|---|---|
| 透明壳种类 Hyaline species | |||
| 尖底类瓮虫 | Amphorellopsis acuta | 2.81 | 8 |
| 卢氏真铃虫 | Eutintinnus lusus-undae | 2.53 | 7 |
| 巴拿马网纹虫 | Favella panamensis | 4.21 | 6 |
| 黏着壳种类 Agglutinated species | |||
| 鲁西塔尼亚类铃虫 | Codonellopsis lusitanica | 1.04 | 8 |
| 运动类铃虫 | C. mobilis | 316.72 | 5 |
| 诺氏薄铃虫 | Leprotintinnus nordqvisti | 0.42 | 7 |
| 简单薄铃虫 | L. simplex | 7.67 | 7 |
| 白领细壳虫 | Stenosemella nivalis | 16.93 | 7 |
| 巴西拟铃虫 | Tintinnopsis brasiliensis | 1.04 | 3 |
| 布氏拟铃虫 | T. butschlii | 1.18 | 7 |
| 清兰拟铃虫 | T. chinglanensis | 65.85 | 7 |
| 有角拟铃虫 | T. corniger | 3.87 | 7 |
| 指状拟铃虫 | T. digita | 9.44 | 8 |
| 直颈拟铃虫 | T. directa | 0.53 | 7 |
| 半旋拟铃虫 | T. hemispiralis | 3.85 | 11 |
| 日本拟铃虫 | T. japonica | 14.21 | 4 |
| 卡拉直克拟铃虫 | T. karajacensis | 0.20 | 3 |
| 罗氏拟铃虫 | T. lohmanni | 6.15 | 7 |
| 梅氏拟铃虫 | T. mayeri | 0.11 | 4 |
| 根状拟铃虫 | T. radix | 29.14 | 7 |
| 圆锥拟铃虫 | T. rapa | 0.59 | 3 |
| 斯氏拟铃虫 | T. schotti | 12.43 | 9 |
| 妥肯丁拟铃虫 | T. tocantinensis | 13.38 | 8 |
| 未定种1 | Tintinnopsis sp.1 | 4.84 | 5 |
| 未定种2 | Tintinnopsis sp.2 | 0.39 | 6 |
| 未定种3 | Tintinnopsis sp.3 | 0.80 | 8 |
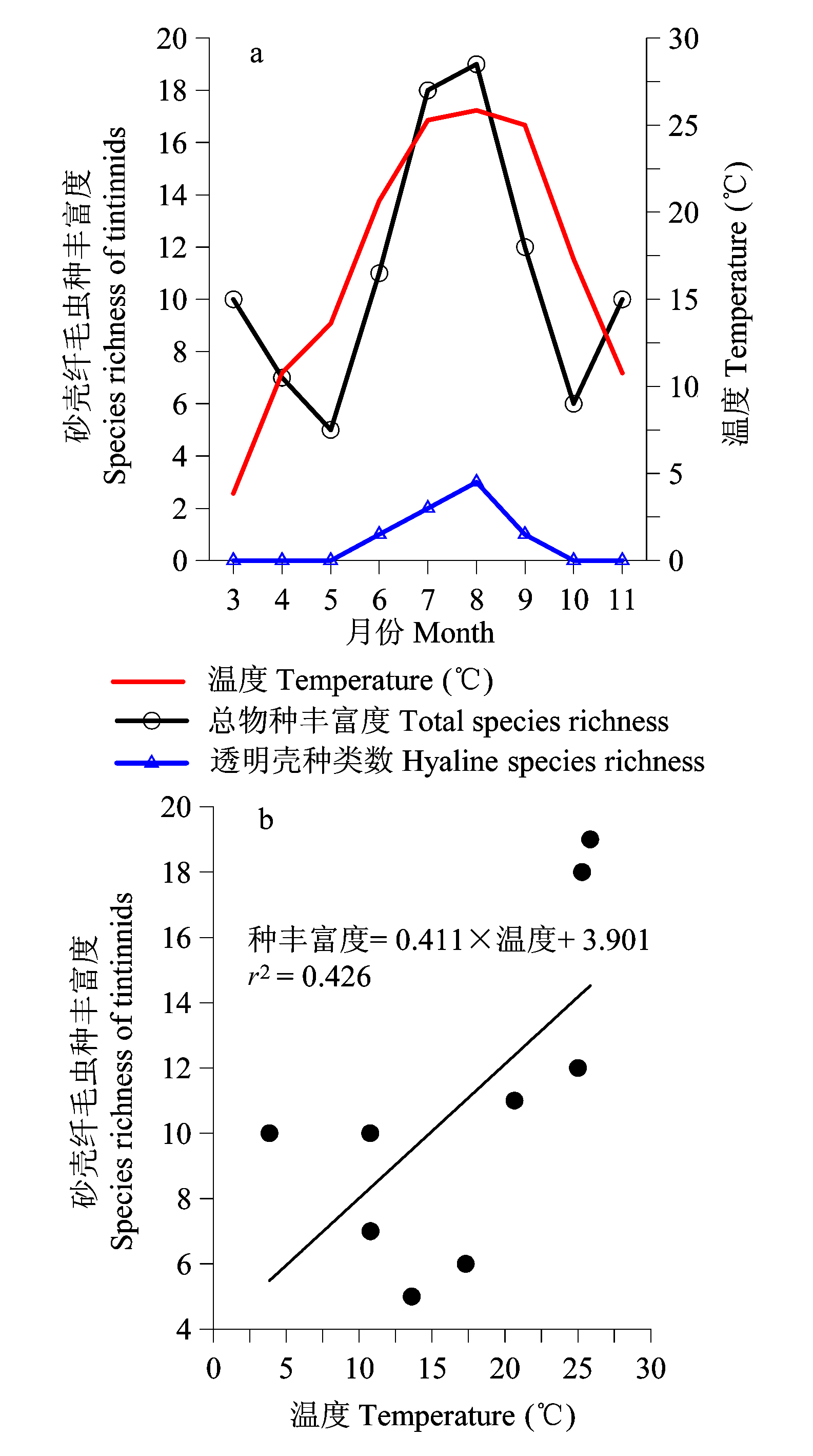
图2 莱州湾3-11月砂壳纤毛虫物种丰富度的变化(a)及温度和物种丰富度的关系(b)
Fig. 2 Variation of species richness of tintinnids in Laizhou Bay from March to November (a), and the relationship between temperature and species richness (b).
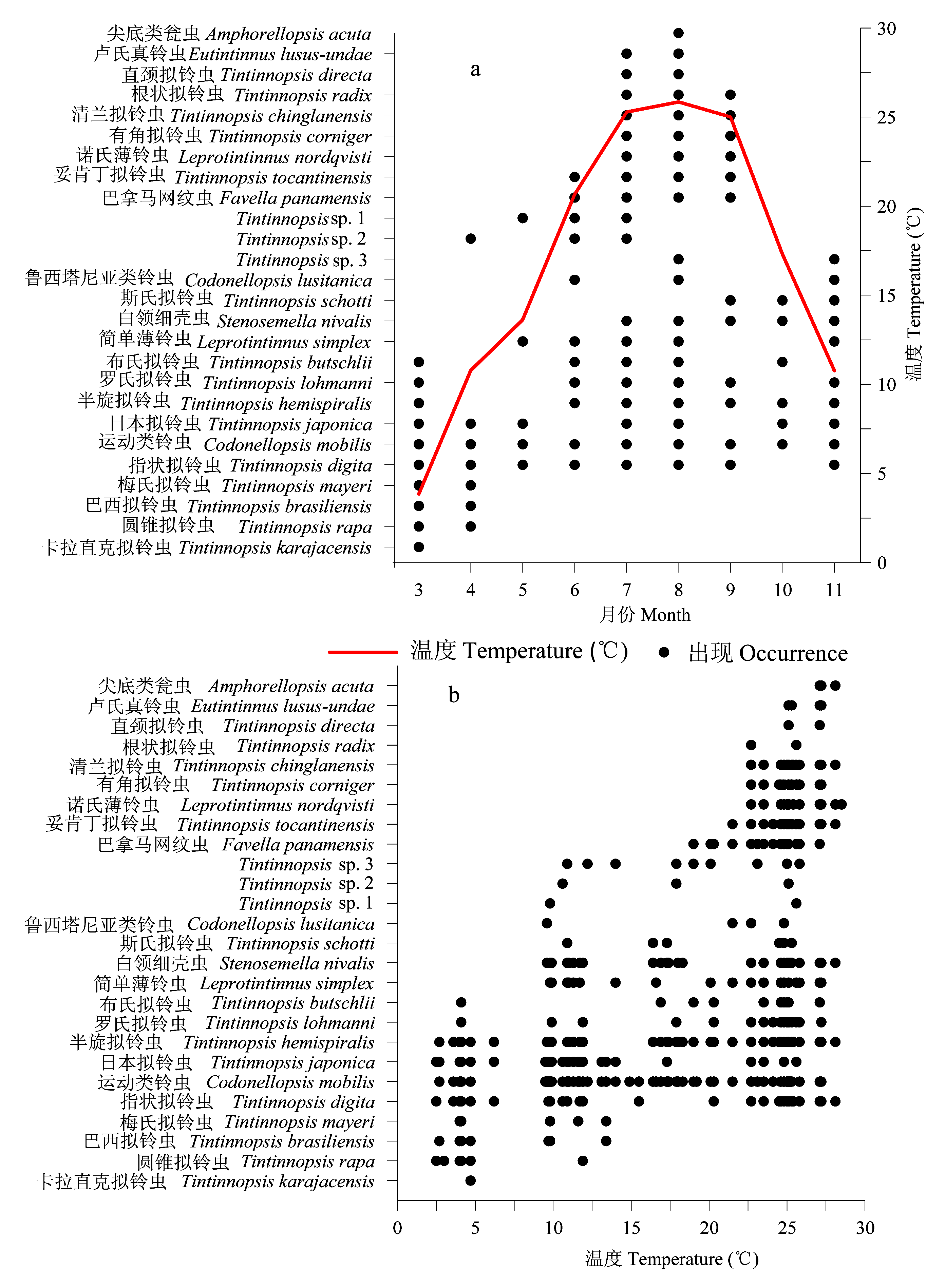
图3 莱州湾3-11月砂壳纤毛虫各种类出现情况(a)及其温度范围(b)
Fig. 3 Temporal variation of temperature (°C), occurrence (a) and temperature range (b) of each tintinnid species in Laizhou Bay from March to November
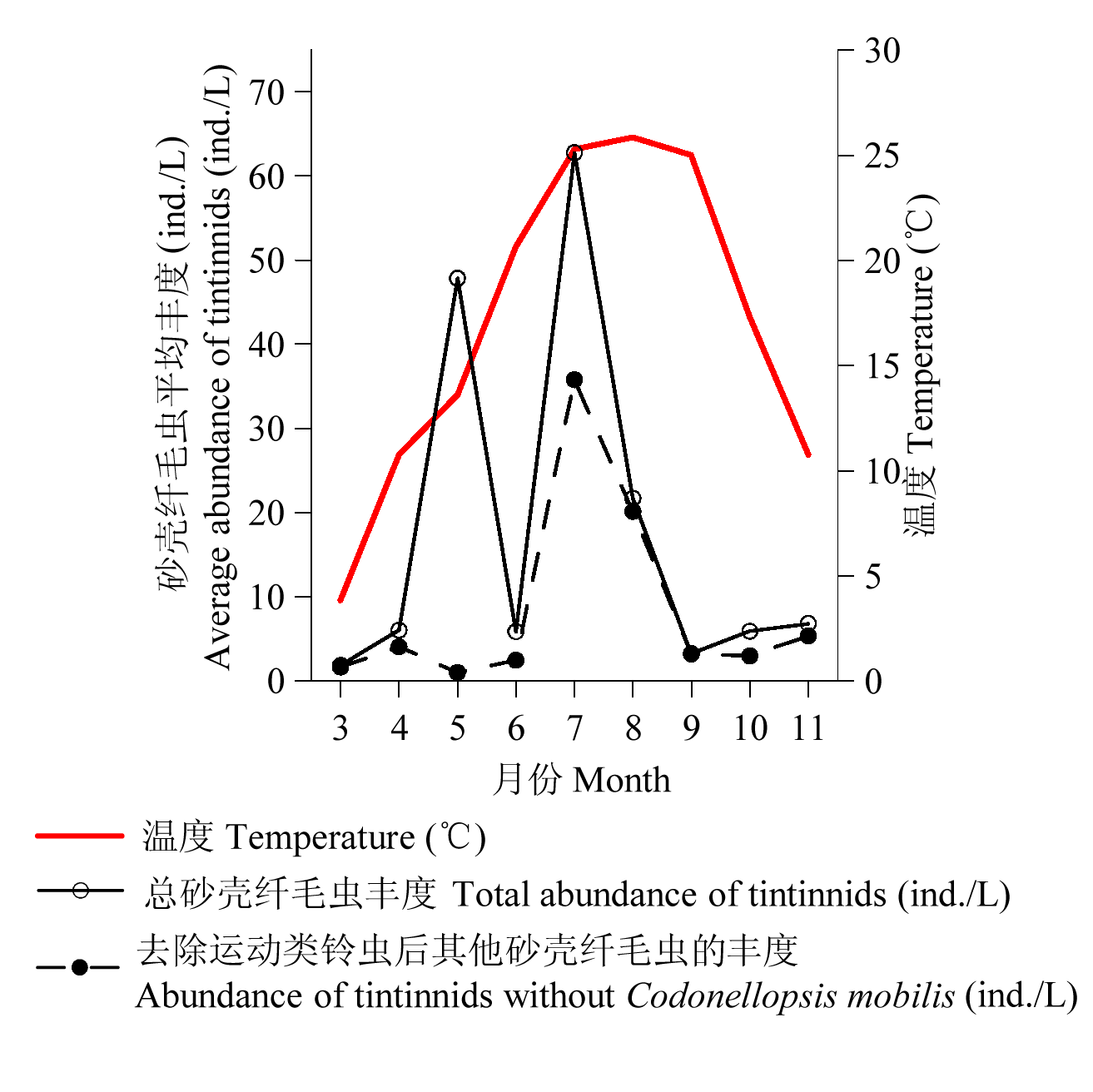
图4 胶州湾3-11月砂壳纤毛虫各站平均丰度(ind./L)的变化
Fig. 4 Temporal variation of temperature (°C), tintinnid average abundance (ind./ L) in Laizhou Bay from March to November.
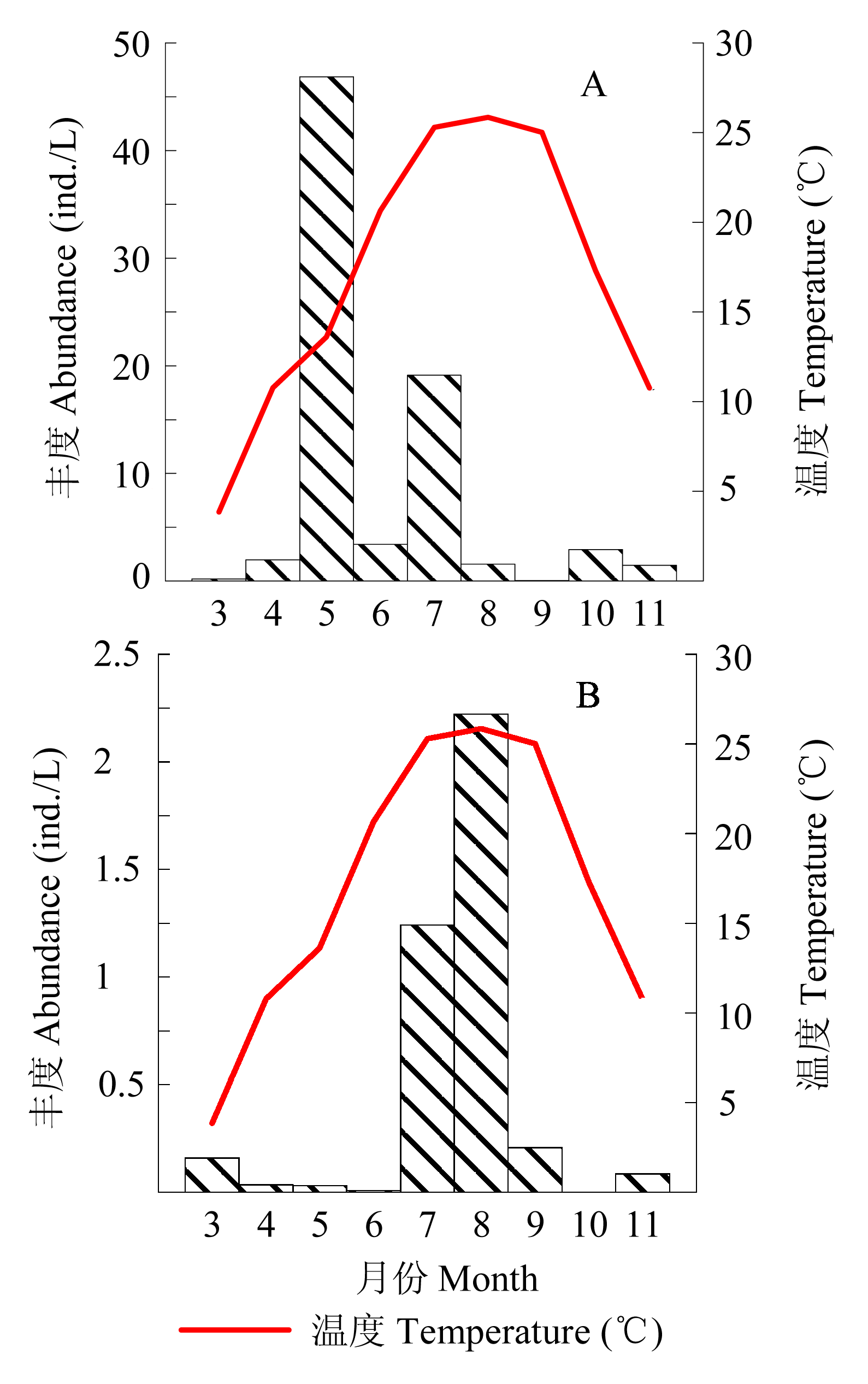
图5 莱州湾3-11月均出现的两种砂壳纤毛虫丰度变化。A: 运动类铃虫, B: 指状拟铃虫。
Fig. 5 Temporal variation of temperature (°C) and tintinnid abundance (ind./L) of 2 species in Laizhou Bay from March to November. A, Codonellopsis mobilis; B, Tintinnopsis digita.
| 优势种 Dominant species | 优势度 Dominance | 优势种 Dominant species | 优势度 Dominance | ||||
|---|---|---|---|---|---|---|---|
| 3月 | 日本拟铃虫 | T. japonica | 0.293 | 8月 | 白领细壳虫 | S. nivalis | 0.179 |
| 半旋拟铃虫 | T. hemispiralis | 0.100 | 清兰拟铃虫 | T. chinglanensis | 0.129 | ||
| 运动类铃虫 | C. mobilis | 0.065 | 妥肯丁拟铃虫 | T. tocantinensis | 0.095 | ||
| 指状拟铃虫 | T. digita | 0.065 | 指状拟铃虫 | T. digita | 0.077 | ||
| 巴西拟铃虫 | T. brasiliensis | 0.065 | 运动类铃虫 | C. mobilis | 0.045 | ||
| 4月 | 日本拟铃虫 | T. japonica | 0.637 | 日本拟铃虫 | T. japonica | 0.044 | |
| 运动类铃虫 | C. mobilis | 0.288 | 罗氏拟铃虫 | T. lohmanni | 0.040 | ||
| 5月 | 运动类铃虫 | C. mobilis | 0.979 | 根状拟铃虫 | T. radix | 0.031 | |
| 9月 | 斯氏拟铃虫 | T. schotti | 0.220 | ||||
| 6月 | 运动类铃虫 | C. mobilis | 0.581 | 有角拟铃虫 | T. corniger | 0.042 | |
| 巴拿马网纹虫 | F. panamensis | 0.189 | 半旋拟铃虫 | T. hemispiralis | 0.033 | ||
| Tintinnopsis sp.1 | 0.049 | 指状拟铃虫 | T. digita | 0.032 | |||
| 7月 | 运动类铃虫 | C. mobilis | 0.305 | 根状拟铃虫 | T. radix | 0.024 | |
| 根状拟铃虫 | T. radix | 0.157 | 10月 | 运动类铃虫 | C. mobilis | 0.496 | |
| 清兰拟铃虫 | T.chinglanensis | 0.086 | 白领细壳虫 | S. nivalis | 0.349 | ||
| 白领细壳虫 | S. nivalis | 0.075 | 11月 | 白领细壳虫 | S. nivalis | 0.445 | |
| 妥肯丁拟铃虫 | T. tocantinensis | 0.067 | 半旋拟铃虫 | T. hemispiralis | 0.267 | ||
| 简单薄铃虫 | L. simplex | 0.033 | 运动类铃虫 | C. mobilis | 0.215 | ||
| 日本拟铃虫 | T. japonica | 0.030 | |||||
表2 莱州湾3-11月砂壳纤毛虫的优势种及其优势度
Table 2 The dominant species of tintinnids and their dominance in Laizhou Bay from March to November
| 优势种 Dominant species | 优势度 Dominance | 优势种 Dominant species | 优势度 Dominance | ||||
|---|---|---|---|---|---|---|---|
| 3月 | 日本拟铃虫 | T. japonica | 0.293 | 8月 | 白领细壳虫 | S. nivalis | 0.179 |
| 半旋拟铃虫 | T. hemispiralis | 0.100 | 清兰拟铃虫 | T. chinglanensis | 0.129 | ||
| 运动类铃虫 | C. mobilis | 0.065 | 妥肯丁拟铃虫 | T. tocantinensis | 0.095 | ||
| 指状拟铃虫 | T. digita | 0.065 | 指状拟铃虫 | T. digita | 0.077 | ||
| 巴西拟铃虫 | T. brasiliensis | 0.065 | 运动类铃虫 | C. mobilis | 0.045 | ||
| 4月 | 日本拟铃虫 | T. japonica | 0.637 | 日本拟铃虫 | T. japonica | 0.044 | |
| 运动类铃虫 | C. mobilis | 0.288 | 罗氏拟铃虫 | T. lohmanni | 0.040 | ||
| 5月 | 运动类铃虫 | C. mobilis | 0.979 | 根状拟铃虫 | T. radix | 0.031 | |
| 9月 | 斯氏拟铃虫 | T. schotti | 0.220 | ||||
| 6月 | 运动类铃虫 | C. mobilis | 0.581 | 有角拟铃虫 | T. corniger | 0.042 | |
| 巴拿马网纹虫 | F. panamensis | 0.189 | 半旋拟铃虫 | T. hemispiralis | 0.033 | ||
| Tintinnopsis sp.1 | 0.049 | 指状拟铃虫 | T. digita | 0.032 | |||
| 7月 | 运动类铃虫 | C. mobilis | 0.305 | 根状拟铃虫 | T. radix | 0.024 | |
| 根状拟铃虫 | T. radix | 0.157 | 10月 | 运动类铃虫 | C. mobilis | 0.496 | |
| 清兰拟铃虫 | T.chinglanensis | 0.086 | 白领细壳虫 | S. nivalis | 0.349 | ||
| 白领细壳虫 | S. nivalis | 0.075 | 11月 | 白领细壳虫 | S. nivalis | 0.445 | |
| 妥肯丁拟铃虫 | T. tocantinensis | 0.067 | 半旋拟铃虫 | T. hemispiralis | 0.267 | ||
| 简单薄铃虫 | L. simplex | 0.033 | 运动类铃虫 | C. mobilis | 0.215 | ||
| 日本拟铃虫 | T. japonica | 0.030 | |||||
| 3月 March | 4月 April | 5月 May | 6月 June | 7月 July | 8月 August | 9月 September | 10月 October | 11月 November | |
|---|---|---|---|---|---|---|---|---|---|
| 种丰富度 Species richness | 10 | 7 | 5 | 11 | 18 | 19 | 12 | 6 | 10 |
| 均匀度指数 Evenness index (J) | 0.78±0.15 | 0.62±0.18 | 0.40±0.30 | 0.59±0.19 | 0.57±0.13 | 0.72±0.08 | 0.76±0.26 | 0.55±0.18 | 0.69±0.11 |
| 多样性指数 Diversity index (H') | 1.71±0.60 | 1.00±0.23 | 0.42±0.29 | 1.33±0.34 | 2.06±0.50 | 2.38±0.32 | 1.45±0.75 | 1.04±0.46 | 1.71±0.36 |
表3 莱州湾3-11月砂壳纤毛虫的种丰富度、均匀度指数和多样性指数
Table 3 Species richness, evenness index and diversity indices of tintinnids in Laizhou Bay from March to November
| 3月 March | 4月 April | 5月 May | 6月 June | 7月 July | 8月 August | 9月 September | 10月 October | 11月 November | |
|---|---|---|---|---|---|---|---|---|---|
| 种丰富度 Species richness | 10 | 7 | 5 | 11 | 18 | 19 | 12 | 6 | 10 |
| 均匀度指数 Evenness index (J) | 0.78±0.15 | 0.62±0.18 | 0.40±0.30 | 0.59±0.19 | 0.57±0.13 | 0.72±0.08 | 0.76±0.26 | 0.55±0.18 | 0.69±0.11 |
| 多样性指数 Diversity index (H') | 1.71±0.60 | 1.00±0.23 | 0.42±0.29 | 1.33±0.34 | 2.06±0.50 | 2.38±0.32 | 1.45±0.75 | 1.04±0.46 | 1.71±0.36 |
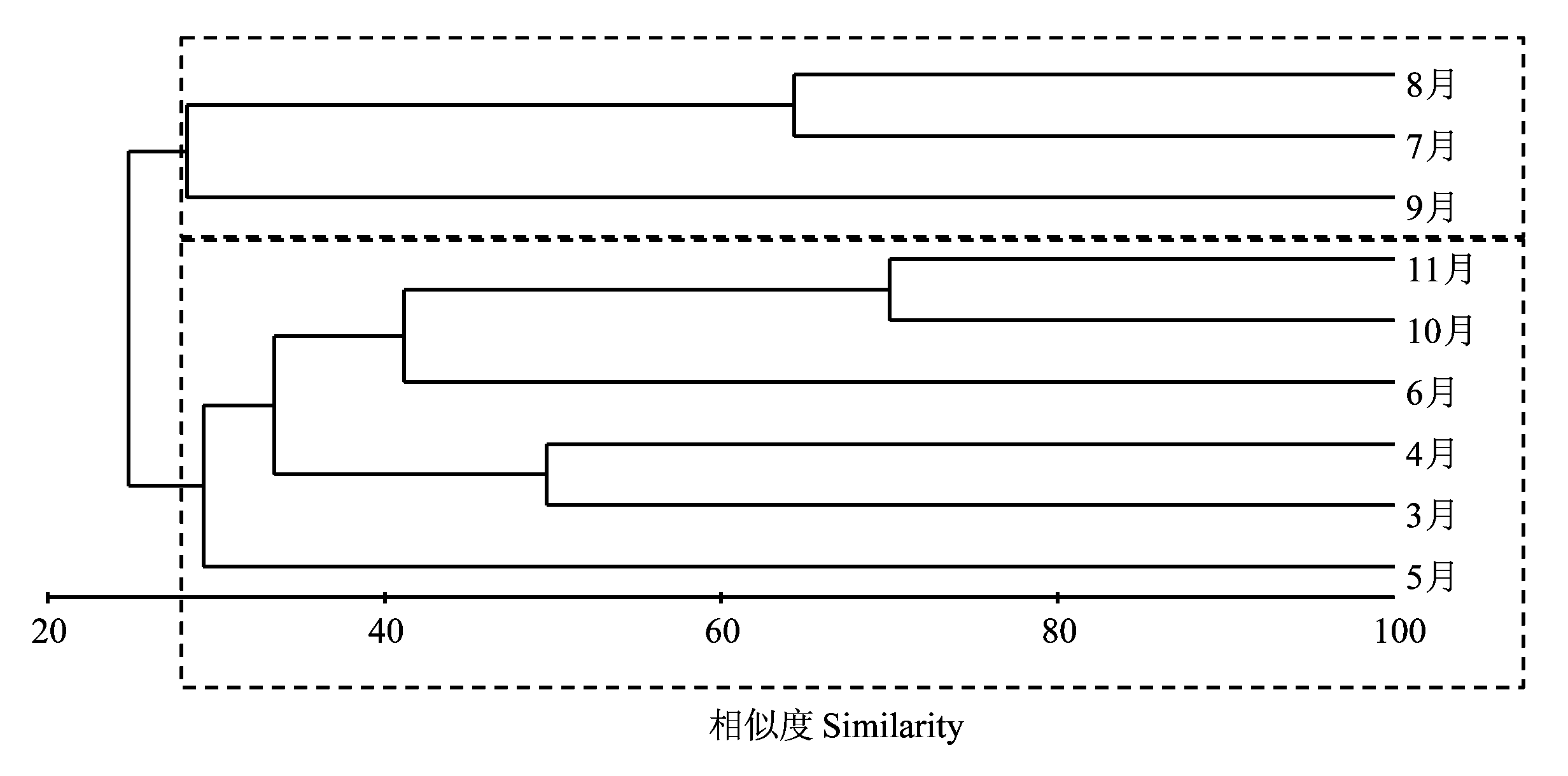
图6 莱州湾3-11月砂壳纤毛虫群落聚类分析结果
Fig. 6 Cluster analysis based on Bray-Curtis similarity matrix of average species abundance (ind./L) of 8 stations in Laizhou Bay from March to November
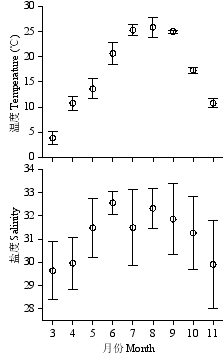
附图 莱州湾3-11月表层温度(°C)和盐度的变化
Fig. S1 Temporal variation of temperature (°C) and salinity in Laizhou Bay from March to November. Data are averages of the 8 stations with standard deviation. http://www.biodiversity-science.net/fileup/PDF/w2013-257-1.pdf
| [1] | .Abboud-Abi Saab M (1989) Distribution and ecology of tintinnids in the plankton of Lebanese coastal waters (eastern Mediterranean). Journal of Plankton Research, 11, 203-222. |
| [2] | .Abboud-Abi Saab M (2002) Annual cycle of the micro- zooplankton communities in the waters surrounding the Palm Island Nature Reserve (north Lebanon), with special attention to tintinnids. Mediterranean Marine Science, 3, 55-76. |
| [3] | .Azam F, Fenchel T, Field JG, Gray JS, Meyer-Reil LA, Thingstad F (1983) The ecological role of water-column microbes in the sea. Marine Ecology Progress Series, 10, 257-263. |
| [4] | .Bojanić N (2001) Seasonal distribution of the ciliated protozoa in Kastela Bay. Journal of the Marine Biological Association of the United Kingdom, 81, 383-390. |
| [5] | .Bojanić N, Šolić M, Krstulović N, Šestanović S, Marasović I, Ninčević Ž (2005) Temporal variability in abundance and biomass of ciliates and copepods in the eutrophicated part of Kaštela Bay (Middle Adriatic Sea). Helgoland Marine Research, 59, 107-120. |
| [6] | .Dolan JR (1991) Guilds of ciliate microzooplankton in the Chesapeake Bay. Estuarine, Coastal and Shelf Science, 33, 137-152. |
| [7] | .Dolan JR, Gallegos CL (2001) Estuarine diversity of tintinnids (planktonic ciliates). Journal of Plankton Research, 23, 1009-1027. |
| [8] | .Dolan JR, Montagnes DJ, Agatha S, Coats DW, Stoecker DK (2013) The Biology and Ecology of Tintinnid Ciliates: Models for Marine Plankton. Wiley-Blackwell, Chichester, UK. |
| [9] | .Elliott DT, Kaufmann RS (2007) Spatial and temporal variability of mesozooplankton and tintinnid ciliates in a seasonally hypersaline estuary. Estuaries and Coasts, 30, 418-430. |
| [10] | .Gold K, Morales EA (1975) Seasonal changes in lorica sizes and the species of Tintinnida in the New York Bight. Journal of Eukaryotic Microbiology, 22, 520-528. |
| [11] | .Graziano C (1989) On the ecology of tintinnids (Ciliophora: Oligotrichida) in the North Irish Sea. Estuarine, Coastal and Shelf Science, 29, 233-245. |
| [12] | .Kamiyama T, Tsujino M (1996) Seasonal variation in the species composition of tintinnid ciliates in Hiroshima Bay, the Seto Inland Sea of Japan. Journal of Plankton Research, 18, 2313-2327. |
| [13] | .Kofoid CA, Campbell AS (1929) A Conspectus of the Marine and Fresh-water Ciliata Belonging to the Suborder Tintinnoinea: with Descriptions of New Species Principally from the Agassiz Expedition to the Eastern Tropical Pacific 1904-1905. University of California, Publications in Zoology, 34, 1-403. |
| [14] | .Kofoid CA, Campbell AS (1939) The Ciliata: the Tintinnoinea. Reports on the Scientific Results of the Expedition to the Eastern Tropical Pacific 1904-1905. Bulletin of the Museum of Comparative Zoology at Harvard College, 84, 1473. |
| [15] | .Laval-Peuto M, Heinbokel JF, Anderson OR, Rassoulzadegan F, Sherr BF (1986) Role of micro- and nanozooplankton in marine food webs. International Journal of Tropical Insect Science, 7, 387-395. |
| [16] | .Leakey R, Burkill PH, Sleigh M (1993) Planktonic ciliates in Southampton Water: quantitative taxonomic studies. Journal of the Marine Biological Association of the United King- dom, 73, 579-594. |
| [17] | .Modigh M, Castaldo S (2002) Variability and persistence in tintinnid assemblages at a Mediterranean coastal site. Aquatic Microbial Ecology, 28, 299-311. |
| [18] | .Montagnes D, Lynn D, Roff J, Taylor W (1988) The annual cycle of heterotrophic planktonic ciliates in the waters surrounding the Isles of Shoals, Gulf of Maine: an assessment of their trophic role. Marine Biology, 99, 21-30. |
| [19] | .Pielou EC (1966) The measurement of diversity in different types of biological collections. Journal of Theoretical Biology, 13, 131-144. |
| [20] | .Pierce RW (1992) Ecology of planktonic ciliates in marine food webs. Reviews in Aquatic Sciences, 6, 139-181. |
| [21] | .Pierce RW, Turner JT (1994) Plankton studies in Buzzards Bay, Massachusetts, USA. IV. Tintinnids, 1987 to 1988. Marine Ecology Progress Series, 112, 235-240. |
| [22] | .Sanders RW (1987) Tintinnids and other microzooplankton: seasonal distributions and relationships to resources and Hydrography in a Maine estuary. Journal of Plankton Research, 9, 65-77. |
| [23] | .Shannon CE, Weaver W (1949) The Mathematical Theory of Communication. University of Illinois Press, Urbana. |
| [24] | .Sitran R, Bergamasco A, Decembrini F, Guglielmo L (2007) Temporal succession of tintinnids in the northern Ionian Sea, central Mediterranean. Journal of Plankton Research, 29, 495-508. |
| [25] | .Sun J (孙军), Liu DY (刘东艳) (2003) The application of diversity indices in marine phytoplankton studies. Acta Oceanologica Sinica(海洋学报), 26, 62-75. (in Chinese with English abstract) |
| [26] | .Urrutxurtu I, Orive E, de la Sota A (2003) Seasonal dynamics of ciliated protozoa and their potential food in an eutrophic estuary (Bay of Biscay). Estuarine, Coastal and Shelf Science, 57, 1169-1182. |
| [27] | .Vaqué D, Blough H, Duarte C (1997) Dynamics of ciliate abundance, biomass and community composition in an oligotrophic coastal environment (NW Mediterranean). Aquatic Microbial Ecology, 12, 71-83. |
| [28] | .Verity PG (1987) Abundance, community composition, size distribution, and production rates of tintinnids in Narragansett Bay, Rhode Island. Estuarine, Coastal and Shelf Science, 24, 671-690. |
| [29] | .Witek M (1998) Annual changes of abundance and biomass of planktonic ciliates in the Gdańsk Basin, southern Baltic. International Review of Hydrobiology, 83, 163-182. |
| [30] | .Xu ZL (徐兆礼), Chen YQ (陈亚瞿) (1989) Aggregated intensity of dominant species of zooplankton in autumn in the East China Sea and Yellow Sea. Chinese Journal of Ecology(生态学杂志), 4, 13-15. (in Chinese with English abstract) |
| [31] | .Yu Y (于莹), Zhang WC (张武昌), Zhao N (赵楠), Sun XX (孙晓霞), Zhang CX (张翠霞), Feng MP (丰美萍), Xiao T (肖天) (2011) Annual variations in the abundance and biomass of planktonic ciliate in the Jiaozhou Bay. Oceanologia et Limnologia Sinica(海洋与湖沼), 42, 690-701. (in Chinese with English abstract) |
| [32] | .Zhang WC (张武昌), Wang R (王荣) (2000) Microzooplank- ton and their grazing pressure on phytoplankton in Bohai Sea. Oceanologia et Limnologia Sinica(海洋与湖沼), 31, 252-258. (in Chinese with English abstract) |
| [33] | .Zhang WC (张武昌), Feng MP (丰美萍), Yu Y (于莹), Zhang CX (张翠霞), Xiao T (肖天) (2012) An Illustrated Guide to Contemporary Tintinnids in the World (砂壳纤毛虫图谱). Science Press, Beijing. (in Chinese) |
| [1] | 吴晓晴 张美惠 葛苏婷 李漫淑 宋坤 沈国春 达良俊 张健. 上海近自然林重建过程中木本植物物种多样性与地上生物量的时空动态——以闵行区生态岛为例[J]. 生物多样性, 2025, 33(5): 24444-. |
| [2] | 张晶晶, 黄文彬, 陈奕廷, 杨泽鹏, 柯伟业, 彭昭杰, 魏世超, 张志伟, 胡怡思, 余文华, 周文良. 广东南澎列岛海洋生态国家级自然保护区造礁石珊瑚多样性及分布特征[J]. 生物多样性, 2025, 33(4): 24424-. |
| [3] | 刘志禹, 吉鑫, 隋国辉, 杨定, 李轩昆. 北京首都国际机场野牛草与杂草草坪无脊椎动物多样性[J]. 生物多样性, 2025, 33(4): 24456-. |
| [4] | 张明燡, 王晓梅, 郑言鑫, 吴楠, 李东浩, 樊恩源, 李娜, 单秀娟, 于涛, 赵春暖, 李波, 徐帅, 吴玉萍, 任利群. 黄河口典型牡蛎礁分布区资源状况和栖息地功能[J]. 生物多样性, 2025, 33(4): 24208-. |
| [5] | 仝淼, 王欢, 张文双, 王超, 宋建潇. 重金属污染土壤中细菌抗生素抗性基因分布特征[J]. 生物多样性, 2025, 33(3): 24101-. |
| [6] | 李艳朋, 陈洁, 卢春洋, 许涵. 海南尖峰岭热带山地雨林64 ha次生林动态监测样地群落结构特征[J]. 生物多样性, 2025, 33(2): 24445-. |
| [7] | 李华亮, 张明军, 张熙斌, 谭荣, 李诗川, 冯尔辉, 林雪云, 陈珉, 颜文博, 曾治高. 海南东寨港国家级自然保护区两栖类群落组成及影响因素[J]. 生物多样性, 2025, 33(2): 24350-. |
| [8] | 王凤琼, 张心怡, 王鑫厅, 姜超, 侯亚丽, 包道日娜. 羊草草原原生群落羊草种群点格局分析[J]. 生物多样性, 2025, 33(2): 24271-. |
| [9] | 弋维, 艾鷖, 吴萌, 田黎明, 泽让东科. 青藏高原高寒草甸土壤古菌群落对不同放牧强度的响应[J]. 生物多样性, 2025, 33(1): 24339-. |
| [10] | 刘源, 杜剑卿, 马丽媛, 杨刚, 田建卿. 纳木措流域岸边带湿地产甲烷古菌群落多样性与分布特征[J]. 生物多样性, 2025, 33(1): 24247-. |
| [11] | 魏诗雨, 宋天骄, 罗佳宜, 张燕, 赵子萱, 茹靖雯, 易华, 林雁冰. 秦岭火地塘针叶林土壤细菌群落的海拔分布格局[J]. 生物多样性, 2024, 32(9): 24180-. |
| [12] | 时永强, 栾青杉, 单秀娟, 韦超, 赵永松, 孙策策, 金显仕. 长岛南部海域浮游动物多样性周年变化[J]. 生物多样性, 2024, 32(7): 23428-. |
| [13] | 程建伟, 徐满厚, 窦永静, 王亚东, 王桠楠, 刘新民, 李永宏. 内蒙古典型草原马粪分解过程中节肢动物群落的季节动态变化[J]. 生物多样性, 2024, 32(6): 24018-. |
| [14] | 郑梦瑶, 李媛, 王雪蓉, 张越, 贾彤. 芦芽山不同植被类型土壤原生动物群落构建机制[J]. 生物多样性, 2024, 32(4): 23419-. |
| [15] | 曲锐, 左振君, 王有鑫, 张良键, 吴志刚, 乔秀娟, 王忠. 基于元素组的生物地球化学生态位及其在不同生态系统中的应用[J]. 生物多样性, 2024, 32(4): 23378-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn