



生物多样性 ›› 2010, Vol. 18 ›› Issue (1): 83-89. DOI: 10.3724/SP.J.1003.2010.083 cstr: 32101.14.SP.J.1003.2010.083
收稿日期:2009-10-20
接受日期:2010-01-17
出版日期:2010-01-20
发布日期:2010-01-20
通讯作者:
叶万辉
作者简介:* E-mail: why@scib.ac.cn基金资助:
Hongping Mu1,2, Yizhu Chen1, Honglin Cao1, Wanhui Ye1,*( )
)
Received:2009-10-20
Accepted:2010-01-17
Online:2010-01-20
Published:2010-01-20
Contact:
Wanhui Ye
摘要:
朱砂根(Ardisia crenata)原产于东亚和东南亚地区, 现已入侵美国南部等地区。为了探讨AM真菌对朱砂根入侵能力的影响, 我们以根段接种的方法扩繁了源自入侵地美国德克萨斯州和原产地广东东莞、四川峨眉山和湖北兴山的朱砂根根系中的AM真菌, 研究了这些不同来源的AM真菌对朱砂根生长和生理状况的影响。结果表明4个不同来源的AM真菌均能够提高朱砂根的叶面积比(LAR)和相对生长速率(RGR), 而对其饱和净光合速率(Pn)、呼吸速率(Rd)、根冠比(R/S)和各器官中氮、磷营养元素含量均没有显著影响。四个不同来源的AM真菌对朱砂根的作用略有差异, 其中入侵地德克萨斯州与原产地广东东莞AM真菌对朱砂根生长的促进作用较强, 但入侵地AM真菌对朱砂根的促进作用并不普遍高于原产地, AM真菌的作用可能并不是导致入侵种群密度远高于本土种群密度的因素。
穆宏平, 陈贻竹, 曹洪麟, 叶万辉 (2010) 不同来源AM真菌对朱砂根生长和生理特征的影响. 生物多样性, 18, 83-89. DOI: 10.3724/SP.J.1003.2010.083.
Hongping Mu, Yizhu Chen, Honglin Cao, Wanhui Ye (2010) Effects of arbuscular mycorrhizal fungi from different sources on the growth and physiology of Ardisia crenata. Biodiversity Science, 18, 83-89. DOI: 10.3724/SP.J.1003.2010.083.
| 来源 Source | 处理 Treatment | 侵染密度Infection density (%) | 侵染率Infection rate (%) |
|---|---|---|---|
| 德克萨斯州 | Inoculated | 59.34 ± 13.92 | 88 ± 6 |
| TX | CK | 0.10 ± 0.04 | 4 ± 2 |
| 东莞 | Inoculated | 63.53 ± 7.13 | 94 ± 2 |
| DG | CK | 1.38 ± 0.51 | 4 ± 2 |
| 兴山 | Inoculated | 65.86 ± 5.00 | 97 ± 3 |
| XS | CK | 0.29 ± 0.24 | 5 ± 3 |
| 峨眉山 | Inoculated | 83.07 ± 4.98 | 98 ± 2 |
| EM | CK | 1.87 ± 0.77 | 6 ± 2 |
表1 不同接种处理条件下朱砂根菌根侵染状况(CK: 对照)
Table 1 Root colonization of Ardisia crenata inoculated with inocula of different sources and controls
| 来源 Source | 处理 Treatment | 侵染密度Infection density (%) | 侵染率Infection rate (%) |
|---|---|---|---|
| 德克萨斯州 | Inoculated | 59.34 ± 13.92 | 88 ± 6 |
| TX | CK | 0.10 ± 0.04 | 4 ± 2 |
| 东莞 | Inoculated | 63.53 ± 7.13 | 94 ± 2 |
| DG | CK | 1.38 ± 0.51 | 4 ± 2 |
| 兴山 | Inoculated | 65.86 ± 5.00 | 97 ± 3 |
| XS | CK | 0.29 ± 0.24 | 5 ± 3 |
| 峨眉山 | Inoculated | 83.07 ± 4.98 | 98 ± 2 |
| EM | CK | 1.87 ± 0.77 | 6 ± 2 |
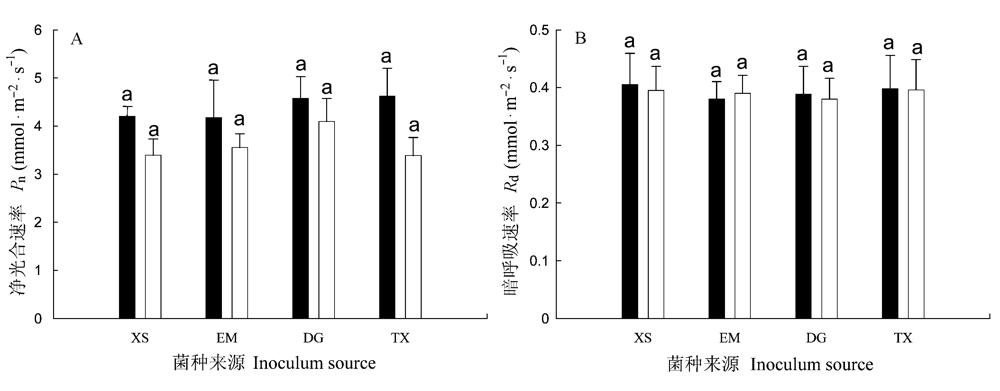
图1 不同接种处理中朱砂根的饱和光下净光合速率(Pn, A)和暗呼吸速率(Rd, B)。■为不同来源AM真菌接种处理, □为相应的对照。误差线表示+1 标准误(s), 同一图中不同小写字母表示不同处理间差异显著(邓肯检验, P<0.05)。
Fig. 1 Light saturated photosynthetic rate (Pn, A) and dark respiration (Rd, B) of Ardisia crenata in response to arbuscular mycorrhizal inocula of different sources. ■ inoculated with inocula of different sources, □ parallel controls. Error bars represent +1 standard error. Different letters in the same figure indicate significant differences (Duncan test, P<0.05).
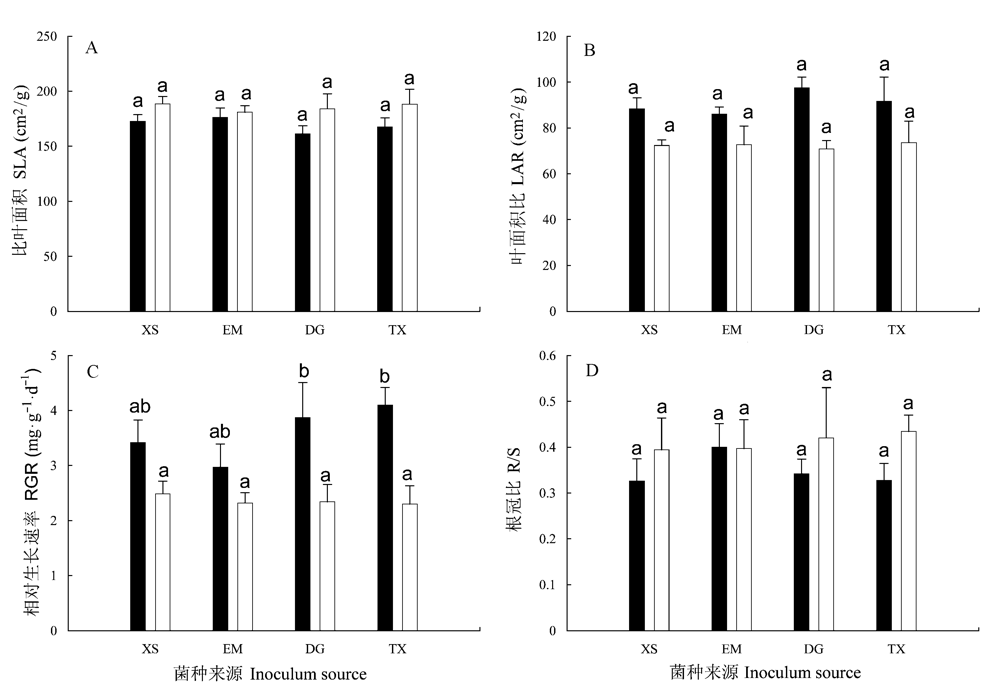
图2 不同接种处理中朱砂根的比叶面积(SLA, A)、叶面积比(LAR, B)、相对生长速率(RGR, C)和根冠比(R/S, D)。■为不同来源AM真菌接种处理, □为相应的对照。误差线表示+1 标准误(s), 同一图中不同小写字母表示不同处理间差异显著(邓肯检验, P<0.05)。
Fig. 2 Specific leaf area (SLA, A), leaf area ratio (LAR, B), relative growth rate (RGR, C) and root:shoot ratio (R/S, D) of Ardisia crenata in response to inocula of different sources. ■ inoculated with inocula of different sources, □ parallel controls. Error bars represent +1 standard error. Different letters in the same figure indicate significant differences (Duncan test, P<0.05).
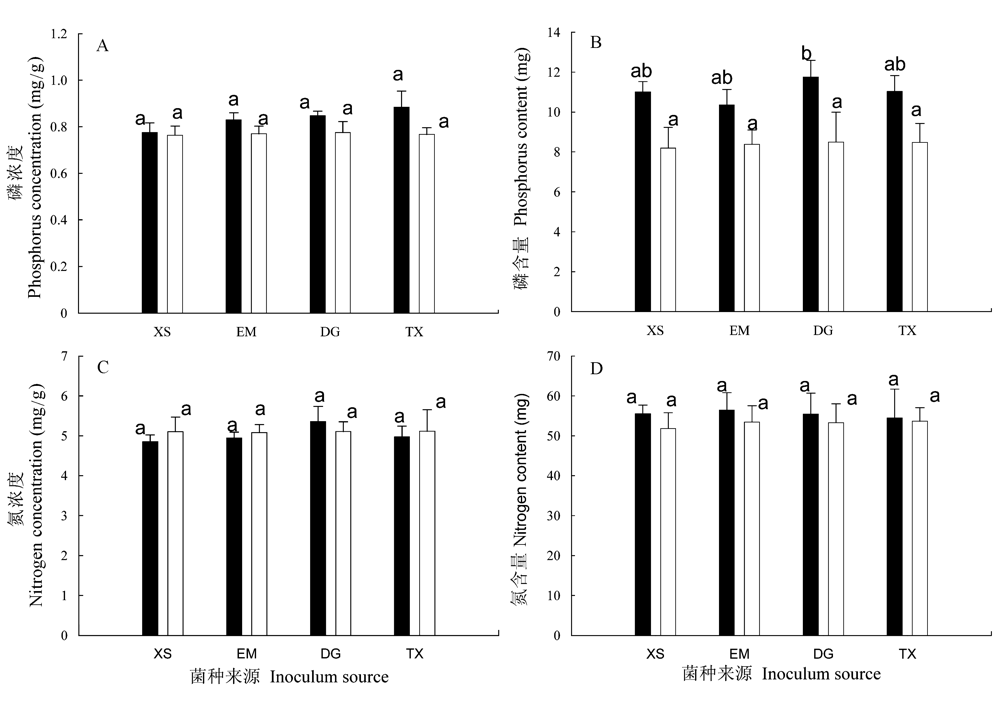
图3 不同接种处理中朱砂根的营养状况: 叶片中组织磷浓度(A)和氮浓度(C)以及植株磷含量(B)和氮含量(D)。■为不同来源AM真菌接种处理, □为相应的对照。误差线表示+1 标准误(s), 同一图中不同小写字母表示不同处理间差异显著(邓肯检验, P<0.05)。
Fig. 3 Phosphorus (A) and nitrogen (C) concentration in leaf and phosphorus (B) and nitrogen (D) content in whole plant of Ardisia crenata in response to inocula of different sources. ■ inoculated with inocula of different sources, □ parallel controls. Error bars represent +1 standard error. Different letters in the same figure indicate significant differences (Duncan test, P<0.05).
| [1] | Abbott LK, Robson AD, De Boer G (1984) The effect of phosphorus on the formation of hyphae in soil by the vesicular-arbuscular mycorrhizal fungus, Glomus fasciculatum. New Phytologist, 97, 437-446. |
| [2] | Bao YY (包玉英) , Sun F (孙芬) (2005) Effect of different inoculum on the isolation of arbuscular mycorrhizal fungi. Acta Scientiarum Naturalium Universitatis Neimongol (内蒙古大学学报), 36, 173-177. (in Chinese with English abstract) |
| [3] | Bray SR, Kitajima K, Sylvia DM (2003) Mycorrhizae differentially alter growth, physiology, and competitive ability of an invasive shrub. Ecological Applications, 13, 565-574. |
| [4] | Callaway RM, Newingham B, Zabinski CA (2001) Compensatory growth and competitive ability of an invasive weed are enhanced by soil fungi and native neighbors. Ecology Letters, 4, 429-433. |
| [5] | Callaway RM, Thelen GC, Barth S, Ramsey PW, Gannon JE (2004) Soil fungi alter interactions between the invader Centaurea maculosa and North American natives. Ecology, 85, 1062-1071. |
| [6] | Carey EV, Marler MJ, Callaway RM (2004) Mycorrhizae transfer carbon from a native grass to an invasive weed: evidence from stable isotopes and physiology. Plant Ecology, 172, 133-141. |
| [7] | Cavender N, Knee M (2006) Relationship of seed source and arbuscular mycorrhizal fungi inoculum type to growth and colonization of big bluestem ( Andropogon gerardii). Plant and Soil, 285, 57-65. |
| [8] | Csurhes S, Edwards R (1998) Potential Environmental Weeds in Australia: Candidate Species for Preventative Control. National Weeds Program, Biodiversity Group, Environment Australia, Canberra. |
| [9] | DeLucia EH, Callaway RM, Thomas EM, Schlesinger WH (1997) Mechanisms of P acquisition for ponderosa pine under different climatic regimes. Annals of Botany, 79, 111-120. |
| [10] | Douds D, Schenck NC (1990) Increased sporulation of vesicular-arbuscular mycorrhizal fungi by manipulation of nutrient regimens. Applied and Environmental Microbiology, 56, 413-418. |
| [11] | Dozier H (1999) Plant Introductions and Invasion: History, Public Awareness, and the Case of Ardisia crenata. PhD dissertation, University of Florida, Florida. |
| [12] | Ehinger M, Koch AM, Sanders IR (2009) Changes in arbuscular mycorrhizal fungal phenotypes and genotypes in response to plant species identity and phosphorus concentration. New Phytologist, 18, 412-423. |
| [13] | Johnson NC, Graham JH, Smith FA (1997) Functioning of mycorrhizal associations along the mutualism-parasitism continuum. New Phytologist, 135, 575-585. |
| [14] | Koch AM, Croll D, Sanders IR (2005) Genetic variability in a population of arbuscular mycorrhizal fungi causes variation in plant growth. Ecology Letters, 9, 103-110. |
| [15] | Koide RT, Li M (1989) Appropriate controls for vesicular-arbuscular mycorrhiza research. New Phytologist, 111, 35-46. |
| [16] |
Landis FC, Gargas A, Givnish TJ (2005) The influence of arbuscular mycorrhizae and light on Wisconsin (USA) sand savanna understories. 2. Plant competition. Mycorrhiza, 15, 555-562.
URL PMID |
| [17] | Liu RJ (刘润进), Li XL(李晓林) (2000) Arbuscular Mycorrhiza and Its Application (AM真菌及其应用). Science Press, Beijing. (in Chinese) |
| [18] | Lorence D, Sussman RW (1988) Diversity, density, and invasion in a Mauritian wet forest. Garden Monographs of the Systematics of the Missouri Botanical Garden, 25, 187-204. |
| [19] | MacDonald IAW, Thébaud C, Strahm WA, Strasberg D (1991) Effects of alien plant invasions on native vegetation remnants on La Reunion (Mascarenes Islands, Indian Ocean). Environmental Conservation, 18, 51-61. |
| [20] | Nijjer S, Rogers WE, Siemann E (2004) The effect of mycorrhizal inoculum on the growth of five native tree species and the invasive Chinese tallow tree ( Sapium sebiferum). Texas Journal of Science, 56, 357-368. |
| [21] | Oppenheimer HL (2004) New Hawaiian plant records for 2003. Bishop Museum Occasional Papers, 79, 8-20. |
| [22] | Philips JM, Hayman DS (1970) Improved procedures for clearing and staining parasistic and vesicular-arbuscular mycorrhizal fungi for rapid assessment of infection. Transactions of the British Mycological Society, 55, 158-161. |
| [23] | Reinhart KO, Callaway RM (2006) Soil biota and invasive plants. New Phytologist, 170, 445-457. |
| [24] | Sharma D, Kapoor R, Bhatnagar AK (2009) Differential growth response of Curculigo orchioides to native arbuscular mycorrhizal fungal (AMF) communities varying in number and fungal components. European Journal of Soil Biology, 45, 328-333. |
| [25] | Singhurst JR, Ledbetter WJ, Holmes WC (1997) Ardisia crenata (Myrsinaceae): new to Texas Southwestern Naturalist, 42, 503-504. |
| [26] | Son CL, Smith SE (1988) Mycorrhizal growth responses: interactions between photon irradiance and phosphorus nutrition. New Phytologist, 108, 305-314. |
| [27] | Streitwolf-Engel R, Boller T, Wiemken A, Sanders IR (1997) Clonal growth traits of two Prunella species are determined by co-occurring arbuscular mycorrhizal fungi from a calcareous grassland. Journal of Ecology, 85, 181-191. |
| [28] | Syvertsen JP, Graham JH (1999) Phosphorus supply and arbuscular mycorrhizas increase growth and net gas exchange responses of two Citrus spp. grown at elevated (CO2). Plant and Soil, 208, 209-219. |
| [29] | van der Heijden EW, Kuyper TW (2001) Does origin of mycorrhizal fungus or mycorrhizal plant influence effectiveness of the mycorrhizal symbiosis? Plant and Soil, 230, 161-174. |
| [30] | van der Heijden MGA, Boller T, Wiemken A, Sanders IR (1998) Different arbuscular mycorrhizal fungal species are potential determinants of plant community structure. Ecology, 79, 2082-2091. |
| [31] | van der Heijden MGA, Wiemken A, Sanders IR (2003) Different arbuscular mycorrhizal fungi alter coexistence and resource distribution between co-occurring plants. New Phytologist, 157, 569-578. |
| [32] | Walling SZ, Zabinski CA (2004) Host plant differences in arbuscular mycorrhizae: extraradical hyphae differences between an invasive forb and a native bunchgrass. Plant and Soil, 265, 335-344. |
| [33] | Weber E (2003) Invasive Plants of the World. CABI Publishing, Wallingford. |
| [34] | Yao XH (姚晓华), Min H (闵航), Yuan HP (袁海平) (2006) Microbial diversity in an acetamiprid-polluted upland soil. Acta Ecologica Sinica (生态学报), 26, 3074-3080. (in Chinese with English abstract) |
| [35] | Zabinski CA, Quinn L, Callaway RM (2002) Phosphorus uptake, not carbon transfer, explains arbuscular mycorrhizal enhancement of Centaurea maculosa in the presence of native grassland species. Functional Ecology, 16, 758-765. |
| [1] | 张浩斌, 肖路, 刘艳杰. 夜间灯光对外来入侵植物和本地植物群落多样性和生长的影响[J]. 生物多样性, 2025, 33(4): 24553-. |
| [2] | 杨向林, 赵彩云, 李俊生, 种方方, 李文金. 植物入侵导致群落谱系结构更加聚集: 以广西国家级自然保护区草本植物为例[J]. 生物多样性, 2024, 32(11): 24175-. |
| [3] | 耿宜佳, 田瑜, 李俊生, 李子圆, 潘玉雪. 《生物多样性公约》框架下外来入侵物种管控的全球进展、挑战和展望[J]. 生物多样性, 2024, 32(11): 24275-. |
| [4] | 龙诗怡, 张博博, 夏宇辰, 费杨帆, 孟亚妮, 吕冰薇, 宋月青, 郑普, 郭陶然, 张健, 黎绍鹏. 本地群落多样性和时间稳定性对加拿大一枝黄花生物量的影响[J]. 生物多样性, 2024, 32(11): 24263-. |
| [5] | 杜聪聪, 冯学宇, 陈志林. 桥头堡效应中气候生态位差异的缩小促进了红火蚁的入侵[J]. 生物多样性, 2024, 32(11): 24276-. |
| [6] | 何林君, 杨文静, 石宇豪, 阿说克者莫, 范钰, 王国严, 李景吉, 石松林, 易桂花, 彭培好. 火烧干扰下植物群落系统发育和功能多样性对紫茎泽兰入侵的影响[J]. 生物多样性, 2024, 32(11): 24269-. |
| [7] | 原雪姣, 张渊媛, 张衍亮, 胡璐祎, 桑卫国, 杨峥, 陈颀. 基于飞机草历史分布数据拟合的物种分布模型及其预测能力[J]. 生物多样性, 2024, 32(11): 24288-. |
| [8] | 韩丽霞, 王永健, 刘宣. 外来物种入侵与本土物种分布区扩张的异同[J]. 生物多样性, 2024, 32(1): 23396-. |
| [9] | 李勇, 李三青, 王欢. 天津野生维管植物编目及分布数据集[J]. 生物多样性, 2023, 31(9): 23128-. |
| [10] | 肖俞, 李宇然, 段禾祥, 任正涛, 冯圣碧, 姜志诚, 李家华, 张品, 胡金明, 耿宇鹏. 高黎贡山外来植物入侵现状及管控建议[J]. 生物多样性, 2023, 31(5): 23011-. |
| [11] | 孙尧初, 潘远飞, 刘木, 潘晓云. 专食性-广食性天敌比例影响入侵植物喜旱莲子草生长防御策略[J]. 生物多样性, 2023, 31(4): 22632-. |
| [12] | 蒲佳佳, 杨平俊, 戴洋, 陶可欣, 高磊, 杜予州, 曹俊, 俞晓平, 杨倩倩. 长江下游外来生物福寿螺的种类及其种群遗传结构[J]. 生物多样性, 2023, 31(3): 22346-. |
| [13] | 沈诗韵, 潘远飞, 陈丽茹, 土艳丽, 潘晓云. 喜旱莲子草原产地和入侵地种群的植物-土壤反馈差异[J]. 生物多样性, 2023, 31(3): 22436-. |
| [14] | 肖巍峰, 左绿荇, 杨文涛, 李朝奎. 基于地理环境相似度的长江经济带入侵物种虚拟负样本生成方法[J]. 生物多样性, 2023, 31(1): 22094-. |
| [15] | 陈敏豪, 张超, 王嘉栋, 湛振杰, 陈君帜, 栾晓峰. 北美水貂和欧亚水獭在东北地区的分布与生态位重叠[J]. 生物多样性, 2023, 31(1): 22289-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn