



生物多样性 ›› 2022, Vol. 30 ›› Issue (12): 22266. DOI: 10.17520/biods.2022266 cstr: 32101.14.biods.2022266
所属专题: 土壤生物与土壤健康
孙翌昕1,2, 李英滨1, 李玉辉1,2, 李冰1,2, 杜晓芳1,*( ), 李琪1,*(
), 李琪1,*( )
)
收稿日期:2022-05-13
接受日期:2022-07-14
出版日期:2022-12-20
发布日期:2022-09-09
通讯作者:
*E-mail: dxf@iae.ac.cn; liq@iae.ac.cn
基金资助:
Yixin Sun1,2, Yingbin Li1, Yuhui Li1,2, Bing Li1,2, Xiaofang Du1,*( ), Qi Li1,*(
), Qi Li1,*( )
)
Received:2022-05-13
Accepted:2022-07-14
Online:2022-12-20
Published:2022-09-09
Contact:
*E-mail: dxf@iae.ac.cn; liq@iae.ac.cn
摘要:
土壤线虫多样性是土壤生态学研究的热点之一, 然而对土壤线虫群落组成及多样性的研究通常受到分类学和方法学的限制。当前, 分子生物学技术的快速发展丰富了我们对土壤线虫多样性的认识, 但也存在一定的局限性。本文综述了常用分子生物学技术如变性梯度凝胶电泳(denaturing gradient gel electrophoresis, DGGE)、末端限制性片段长度多态性分析(terminal restriction fragment length polymorphism, T-RFLP)、实时荧光定量PCR (quantitative real-time PCR, qPCR)和高通量测序(high-throughput sequencing, HTS)技术近年来在线虫多样性研究中的应用, 重点从土壤线虫DNA提取方法、引物和数据库的选择、高通量测序技术和形态学鉴定结果的比较等方面阐述了高通量测序技术在线虫多样性研究中的优势与不足, 并提出选择合适的线虫DNA提取方法结合特定引物和数据库进行注释分析, 仍是今后使用高通量测序技术开展线虫多样性研究的重点。当研究目标是土壤线虫多样性时, 优先推荐富集线虫悬液提取DNA的方法, 因此, 研究人员应根据具体目标选择最优组合开展实验研究。
孙翌昕, 李英滨, 李玉辉, 李冰, 杜晓芳, 李琪 (2022) 高通量测序技术在线虫多样性研究中的应用. 生物多样性, 30, 22266. DOI: 10.17520/biods.2022266.
Yixin Sun, Yingbin Li, Yuhui Li, Bing Li, Xiaofang Du, Qi Li (2022) Application of high-throughput sequencing technique in the study of nematode diversity. Biodiversity Science, 30, 22266. DOI: 10.17520/biods.2022266.
| 分子生物学技术 Molecular biology techniques | 优点 Advantages | 缺点 Disadvantages | 主要文献 References |
|---|---|---|---|
| 变性梯度凝胶电泳 Denaturing gradient gel electrophoresis (DGGE) | 相比形态学鉴定简单快速, 提高物种鉴定速度和时间成本效益 Compared with morphological identification, it is simple and rapid, and can improve the speed and time cost effectiveness of species identification | 灵敏度不高, 不适合测量群落多样性; 在线虫稀有物种的代表性上鉴定不充分; 不能够直接评估物种丰富度 Low sensitivity, not suitable for measuring community diversity; the representation of rare nematode species was not adequately identified; species richness cannot be directly assessed | 1993; 2005; 2008; 2013 |
| 末端限制性片段长度多态性分析 Terminal restriction fragment length polymorphism (T-RFLP) | 操作简单; 有利于处理大量样本; 成本相对较低, 可以跨电泳运行比较数据 The manipulation is simple and good for processing a large number of samples; the cost is relatively low and data can be compared across electrophoresis runs | 不能准确反映线虫群落结构组成, 结果有一定偏差, 可能高估线虫群落的多样性 The nematode community composition could not be accurately reflected, and the results may overestimate the diversity of nematode communities | 2012; 2014; 2015 |
| 实时荧光定量PCR Quantitative real-time PCR (qPCR) | 灵敏度高、特异性强; 检测结果可定量; 适用于大量样本; 可以确定特定线虫类群丰度 High sensitivity and specificity; the detection results can be quantified; suitable for large samples; the abundance of specific nematode trophic groups can be determined | 不能提供样本中所有线虫群落的多样性信息, 无法计算线虫的富集指数和通道指数; 对引物要求较高, 容易出现非特异性条带; 成本也较高 The diversity information of all nematode communities in the samples could not be provided, and the enrichment index (EI) and channel index (CI) of nematode communities could not be calculated; high requirements for primers, prone to non-specific bands; the cost is higher | 2006; 2010; 2010; 2012 |
| 高通量测序 High-throughput sequencing (HTS) | 成本低; 测序量大、灵敏度高、用时短; 提供更高的分类学分辨率 Lower in cost; large amount in sequencing, high sensitivity and short time; provides higher taxonomic resolution | 只能以相对丰度表征物种数量; 现有引物不是线虫特异性引物; 线虫参考数据库有待充实 Species numbers can only be represented by relative abundance; existing primers are not nematode-specific primers; the nematode reference database needs to be enriched | 2017; 2018; 2020; 2021 |
表1 不同分子生物学技术分析线虫多样性的优缺点
Table 1 Advantages and disadvantages of nematode diversity analysis using different molecular biology techniques
| 分子生物学技术 Molecular biology techniques | 优点 Advantages | 缺点 Disadvantages | 主要文献 References |
|---|---|---|---|
| 变性梯度凝胶电泳 Denaturing gradient gel electrophoresis (DGGE) | 相比形态学鉴定简单快速, 提高物种鉴定速度和时间成本效益 Compared with morphological identification, it is simple and rapid, and can improve the speed and time cost effectiveness of species identification | 灵敏度不高, 不适合测量群落多样性; 在线虫稀有物种的代表性上鉴定不充分; 不能够直接评估物种丰富度 Low sensitivity, not suitable for measuring community diversity; the representation of rare nematode species was not adequately identified; species richness cannot be directly assessed | 1993; 2005; 2008; 2013 |
| 末端限制性片段长度多态性分析 Terminal restriction fragment length polymorphism (T-RFLP) | 操作简单; 有利于处理大量样本; 成本相对较低, 可以跨电泳运行比较数据 The manipulation is simple and good for processing a large number of samples; the cost is relatively low and data can be compared across electrophoresis runs | 不能准确反映线虫群落结构组成, 结果有一定偏差, 可能高估线虫群落的多样性 The nematode community composition could not be accurately reflected, and the results may overestimate the diversity of nematode communities | 2012; 2014; 2015 |
| 实时荧光定量PCR Quantitative real-time PCR (qPCR) | 灵敏度高、特异性强; 检测结果可定量; 适用于大量样本; 可以确定特定线虫类群丰度 High sensitivity and specificity; the detection results can be quantified; suitable for large samples; the abundance of specific nematode trophic groups can be determined | 不能提供样本中所有线虫群落的多样性信息, 无法计算线虫的富集指数和通道指数; 对引物要求较高, 容易出现非特异性条带; 成本也较高 The diversity information of all nematode communities in the samples could not be provided, and the enrichment index (EI) and channel index (CI) of nematode communities could not be calculated; high requirements for primers, prone to non-specific bands; the cost is higher | 2006; 2010; 2010; 2012 |
| 高通量测序 High-throughput sequencing (HTS) | 成本低; 测序量大、灵敏度高、用时短; 提供更高的分类学分辨率 Lower in cost; large amount in sequencing, high sensitivity and short time; provides higher taxonomic resolution | 只能以相对丰度表征物种数量; 现有引物不是线虫特异性引物; 线虫参考数据库有待充实 Species numbers can only be represented by relative abundance; existing primers are not nematode-specific primers; the nematode reference database needs to be enriched | 2017; 2018; 2020; 2021 |
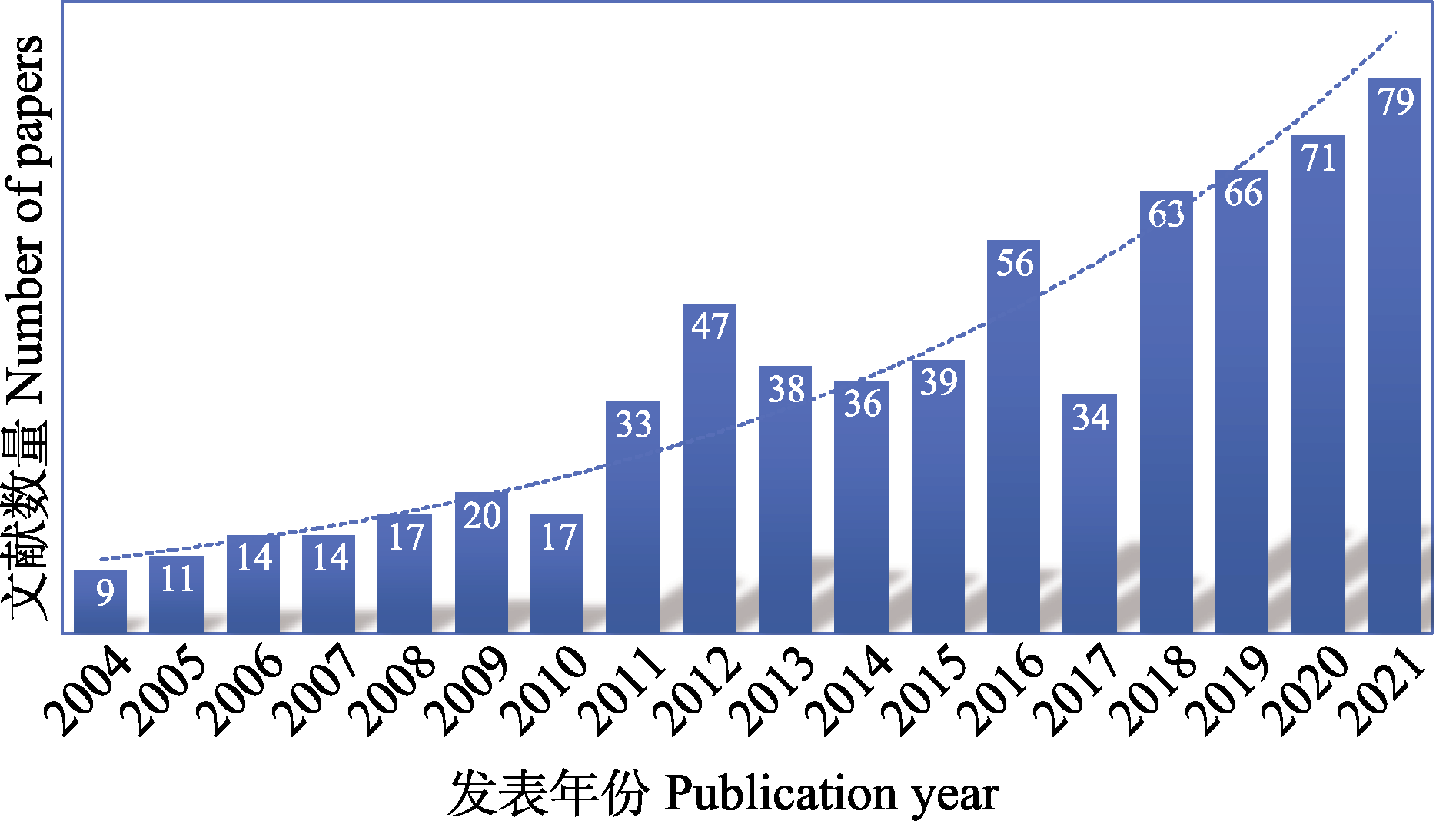
图1 近18年(2004-2021年)利用高通量测序技术开展线虫多样性研究的发文数量(数据来源: Web of Science)
Fig. 1 Number of publications related to nematode diversity using high-throughput sequencing technology in recent 18 years (2004-2021)
| 提取方法 Extraction method | 优点 Advantages | 缺点 Disadvantages | 土壤样品量 Soil sample weight (g) | 线虫提取试剂盒 Nematode DNA extraction kit | 文献 References |
|---|---|---|---|---|---|
| 土壤中提取线 虫DNA Nematode DNA was extracted from soil | 无需富集步骤, 样本量较大时减小工作量 No enrichment step, suitable for work with large sample size | 适合土壤线虫的土壤试剂盒数量少, 且结果不稳定, 在不同的实验条件下影响试剂盒的提取成功率 The kinds of soil kit suitable for soil nematodes were few; the extraction efficiency is instable and affected by experimental conditions | 0.25 | PowerLyzerTM PowerSoil? DNA Isolation Kit | 2015 |
| 0.25 | PowerLyzer Soil DNA extraction kit | 2020 | |||
| 10 | PowerMax Soil DNA isolation kit | 2018 | |||
| 10 | DNeasy PowerSoil Kit | 2021 | |||
| 25 | NucleoSpin? Soil | 2017 | |||
| Precellys? Soil DNA Kit | |||||
| PowerLyzer? Soil DNA Isolation Kit | |||||
| PowerSoil? Soil DNA Isolation Kit | |||||
| PowerMax? Soil DNA Isolation Kit | |||||
| E.Z.N.A. Mag-Bind? Soil | |||||
| 富集线虫悬液 提取DNA Nematode DNA was extracted from the enriched suspension | 避免了放大其他物种(如真菌和植物)的DNA Avoiding amplification of other species DNA, such as fungi and plants | 费时费力, 可能会偏向于线虫的特定属或发育阶段 Prefer to some particular genus or developmental stage of nematodes 在处理大量样品时, 富集工作易成为瓶颈 Enrichment process is a restriction when handling large number of samples | 100 | Clear Detections Nematode DNA extraction and purification kit? | 2018 |
| 40 | MO BIO UltraClean? Tissue & Cells DNA Isolation kit | 2018 | |||
| 100 | DNeasy Blood & Tissue Kit | 2020; 2022 |
表2 不同试剂盒技术及DNA提取方法比较
Table 2 Comparison of different kit techniques and DNA extraction methods
| 提取方法 Extraction method | 优点 Advantages | 缺点 Disadvantages | 土壤样品量 Soil sample weight (g) | 线虫提取试剂盒 Nematode DNA extraction kit | 文献 References |
|---|---|---|---|---|---|
| 土壤中提取线 虫DNA Nematode DNA was extracted from soil | 无需富集步骤, 样本量较大时减小工作量 No enrichment step, suitable for work with large sample size | 适合土壤线虫的土壤试剂盒数量少, 且结果不稳定, 在不同的实验条件下影响试剂盒的提取成功率 The kinds of soil kit suitable for soil nematodes were few; the extraction efficiency is instable and affected by experimental conditions | 0.25 | PowerLyzerTM PowerSoil? DNA Isolation Kit | 2015 |
| 0.25 | PowerLyzer Soil DNA extraction kit | 2020 | |||
| 10 | PowerMax Soil DNA isolation kit | 2018 | |||
| 10 | DNeasy PowerSoil Kit | 2021 | |||
| 25 | NucleoSpin? Soil | 2017 | |||
| Precellys? Soil DNA Kit | |||||
| PowerLyzer? Soil DNA Isolation Kit | |||||
| PowerSoil? Soil DNA Isolation Kit | |||||
| PowerMax? Soil DNA Isolation Kit | |||||
| E.Z.N.A. Mag-Bind? Soil | |||||
| 富集线虫悬液 提取DNA Nematode DNA was extracted from the enriched suspension | 避免了放大其他物种(如真菌和植物)的DNA Avoiding amplification of other species DNA, such as fungi and plants | 费时费力, 可能会偏向于线虫的特定属或发育阶段 Prefer to some particular genus or developmental stage of nematodes 在处理大量样品时, 富集工作易成为瓶颈 Enrichment process is a restriction when handling large number of samples | 100 | Clear Detections Nematode DNA extraction and purification kit? | 2018 |
| 40 | MO BIO UltraClean? Tissue & Cells DNA Isolation kit | 2018 | |||
| 100 | DNeasy Blood & Tissue Kit | 2020; 2022 |
| 线虫提取方式 Nematode extraction method | 试剂盒 Nematode DNA extraction kit | 引物 Primers | 数据库 Databases | 与形态学结果比较 Comparison with morphological results | 文献 References | |||
|---|---|---|---|---|---|---|---|---|
| 形态学 Morphology | 高通量测序 HTS | 共有 Common | 营养类群 Trophic groups | |||||
| 土壤中提取线虫DNA Nematode DNA was extracted from soil | PowerSoil DNA Isolation Kit (MoBioLaboratories, Carlsbad, CA, USA) | 3NDf/ 1132-rmodR | NCBI SRA database | 27属 27 genera | 42属 42 genera | 15属 15 genera | HTS更偏向植物寄生线虫 HTS prefers to plant parasitic nematodes | 2021 |
| 富集线虫悬液提取DNA Nematode DNA was extracted from the enriched suspension | MO BIO UltraClean? Tissue & Cells DNA Isolation kit | NF1F/18Sr2bR | The SILVA 111 database | 21科 21 families | 30科 30 families | - | HTS偏向食细菌线虫, 形态学偏向食真菌线虫 HTS prefers to bacterivores nematodes; morphology prefers to fungivores nematodes | 2018 |
| The Clear Detections Nematode DNA extraction and purification kit? (Clear Detections, Wageningen, the Netherlands) | 3NDf/1132r | PR2 database | 71属 71 genera | 101属 101 genera | - | HTS与形态学更偏向植物寄生线虫 HTS and morphology prefer to plant parasitic nematodes | 2018 | |
| DNeasy Blood & Tissue Kit | NF1F/18Sr2bR | NCBI NT database | 81属 81 genera | 77属 77 genera | 36属 36 genera | HTS与形态学更偏向植物寄生线虫 HTS and morphology prefer to plant parasitic nematodes | 2020 | |
| Ion Torrent PGM with the Ion PGM Hi-Q Sequencing Kit and the Ion 318 Chip Kit (Life Technologies) | NF1F/18Sr2bR | SILVA 132 eukaryotic database | 46属 46 genera | 51属 51 genera | 27属 27 genera | HTS中食细菌线虫和杂食性线虫的相对丰度高于形态学鉴定 The relative abundance of bacterivores nematodes and omnivorous nematodes in HTS was higher than morphological identification | 2022 | |
表3 高通量测序(HTS)技术和形态学结果比较
Table 3 Comparison of high-throughput sequencing (HTS) technology and morphological results
| 线虫提取方式 Nematode extraction method | 试剂盒 Nematode DNA extraction kit | 引物 Primers | 数据库 Databases | 与形态学结果比较 Comparison with morphological results | 文献 References | |||
|---|---|---|---|---|---|---|---|---|
| 形态学 Morphology | 高通量测序 HTS | 共有 Common | 营养类群 Trophic groups | |||||
| 土壤中提取线虫DNA Nematode DNA was extracted from soil | PowerSoil DNA Isolation Kit (MoBioLaboratories, Carlsbad, CA, USA) | 3NDf/ 1132-rmodR | NCBI SRA database | 27属 27 genera | 42属 42 genera | 15属 15 genera | HTS更偏向植物寄生线虫 HTS prefers to plant parasitic nematodes | 2021 |
| 富集线虫悬液提取DNA Nematode DNA was extracted from the enriched suspension | MO BIO UltraClean? Tissue & Cells DNA Isolation kit | NF1F/18Sr2bR | The SILVA 111 database | 21科 21 families | 30科 30 families | - | HTS偏向食细菌线虫, 形态学偏向食真菌线虫 HTS prefers to bacterivores nematodes; morphology prefers to fungivores nematodes | 2018 |
| The Clear Detections Nematode DNA extraction and purification kit? (Clear Detections, Wageningen, the Netherlands) | 3NDf/1132r | PR2 database | 71属 71 genera | 101属 101 genera | - | HTS与形态学更偏向植物寄生线虫 HTS and morphology prefer to plant parasitic nematodes | 2018 | |
| DNeasy Blood & Tissue Kit | NF1F/18Sr2bR | NCBI NT database | 81属 81 genera | 77属 77 genera | 36属 36 genera | HTS与形态学更偏向植物寄生线虫 HTS and morphology prefer to plant parasitic nematodes | 2020 | |
| Ion Torrent PGM with the Ion PGM Hi-Q Sequencing Kit and the Ion 318 Chip Kit (Life Technologies) | NF1F/18Sr2bR | SILVA 132 eukaryotic database | 46属 46 genera | 51属 51 genera | 27属 27 genera | HTS中食细菌线虫和杂食性线虫的相对丰度高于形态学鉴定 The relative abundance of bacterivores nematodes and omnivorous nematodes in HTS was higher than morphological identification | 2022 | |
| [1] |
Abad D, Albaina A, Aguirre M, Laza-Martínez A, Uriarte I, Iriarte A, Villate F, Estonba A (2016) Is metabarcoding suitable for estuarine plankton monitoring? A comparative study with microscopy. Marine Biology, 163, 1-13.
DOI URL |
| [2] | Ahmed M, Back MA, Prior T, Karssen G, Lawson R, Adams I, Sapp M (2019) Metabarcoding of soil nematodes: The importance of taxonomic coverage and availability of reference sequences in choosing suitable marker(s). Metabarcoding and Metagenomics, 3, 77-99. |
| [3] |
Bhadury P, Austen MC, Bilton DT, Lambshead PJD, Rogers AD, Smerdon GR (2005) Combined morphological and molecular analysis of individual nematodes through short-term preservation in formalin. Molecular Ecology Notes, 5, 965-968.
DOI URL |
| [4] |
Chen XY, Daniell TJ, Neilson R, O’Flaherty V, Griffiths BS (2010) A comparison of molecular methods for monitoring soil nematodes and their use as biological indicators. European Journal of Soil Biology, 46, 319-324.
DOI URL |
| [5] |
Cook AA, Bhadury P, Debenham NJ, Meldal BHM, Blaxter ML, Smerdon GR, Austen MC, Lambshead PJD, Rogers AD (2005) Denaturing gradient gel electrophoresis (DGGE) as a tool for identification of marine nematodes. Marine Ecology Progress Series, 291, 103-113.
DOI URL |
| [6] |
Creer S, Fonseca VG, Porazinska DL, Giblin-Davis RM, Sung W, Power DM, Packer M, Carvalho GR, Blaxter ML, Lambshead PJD, Thomas WK (2010) Ultrasequencing of the meiofaunal biosphere: Practice, pitfalls and promises. Molecular Ecology, 19, 4-20.
DOI URL |
| [7] |
Donn S, Neilson R, Griffiths BS, Daniell TJ (2012) A novel molecular approach for rapid assessment of soil nematode assemblages—Variation, validation and potential applications. Methods in Ecology and Evolution, 3, 12-23.
DOI URL |
| [8] |
Drummond AJ, Newcomb RD, Buckley TR, Xie D, Dopheide A, Potter BCM, Heled J, Ross HA, Tooman L, Grosser S, Park D, Demetras NJ, Stevens MI, Russell JC, Anderson SH, Carter A, Nelson N (2015) Evaluating a multigene environmental DNA approach for biodiversity assessment. GigaScience, 4, 46.
DOI PMID |
| [9] |
Du XF, Li YB, Han X, Ahmad W, Li Q (2020) Using high-throughput sequencing quantitatively to investigate soil nematode community composition in a steppe-forest ecotone. Applied Soil Ecology, 152, 103562.
DOI URL |
| [10] | Du XF, Li YB, Liu F, Su XL, Li Q (2018) Structure and ecological functions of soil micro-food web. Chinese Journal of Applied Ecology, 29, 403-411. (in Chinese with English abstract) |
|
[ 杜晓芳, 李英滨, 刘芳, 宿晓琳, 李琪 (2018) 土壤微食物网结构与生态功能. 应用生态学报, 29, 403-411.]
DOI |
|
| [11] | Du XF, Liang WJ, Li Q (2021) DNA extraction, amplification and high-throughput sequencing of soil nematode community. Microbiome Protocols eBook, Bio-101, e2104085. (in Chinese) |
| [ 杜晓芳, 梁文举, 李琪 (2021) 土壤线虫群落DNA提取、扩增及高通量测序. 微生物组实验手册, Bio-101, e2104085.] | |
| [12] |
Du XF, Liu HW, Li YB, Li B, Han X, Li YH, Mahamood M, Li Q (2022) Soil community richness and composition jointly influence the multifunctionality of soil along the forest-steppe ecotone. Ecological Indicators, 139, 108900.
DOI URL |
| [13] |
Edel-Hermann V, Gautheron N, Alabouvette C, Steinberg C (2008) Fingerprinting methods to approach multitrophic interactions among microflora and microfauna communities in soil. Biology and Fertility of Soils, 44, 975-984.
DOI URL |
| [14] |
Foucher A, Bongers T, Noble LR, Wilson M (2004) Assessment of nematode biodiversity using DGGE of 18S rDNA following extraction of nematodes from soil. Soil Biology and Biochemistry, 36, 2027-2032.
DOI URL |
| [15] |
Foucher A, Wilson M (2002) Development of a polymerase chain reaction-based denaturing gradient gel electrophoresis technique to study nematode species biodiversity using the 18s rDNA gene. Molecular Ecology Notes, 2, 45-48.
DOI URL |
| [16] |
Gao DD, Moreira-Grez B, Wang KL, Zhang W, Xiao SS, Wang WL, Chen HS, Zhao J (2021) Effects of ecosystem disturbance on nematode communities in calcareous and red soils: Comparison of taxonomic methods. Soil Biology and Biochemistry, 155, 108162.
DOI URL |
| [17] |
Geisen S, Snoek LB ten Hooven FC, Duyts H, Kostenko O, Bloem J, Martens H, Quist CW, Helder JA,van der Putten WH (2018) Integrating quantitative morphological and qualitative molecular methods to analyse soil nematode community responses to plant range expansion. Methods in Ecology and Evolution, 9, 1366-1378.
DOI URL |
| [18] |
George PBL, Lindo Z (2015) Congruence of community structure between taxonomic identification and T-RFLP analyses in free-living soil nematodes. Pedobiologia, 58, 113-117.
DOI URL |
| [19] |
Griffiths BS, de Groot GA, Laros I, Stone D, Geisen S (2018) The need for standardisation: Exemplified by a description of the diversity, community structure and ecological indices of soil nematodes. Ecological Indicators, 87, 43-46.
DOI URL |
| [20] |
Griffiths BS, Donn S, Neilson R, Daniell TJ (2006) Molecular sequencing and morphological analysis of a nematode community. Applied Soil Ecology, 32, 325-337.
DOI URL |
| [21] |
Holeva R, Phillips MS, Neilson R, Brown DJF, Young V, Boutsika K, Blok VC (2006) Real-time PCR detection and quantification of vector trichodorid nematodes and tobacco rattle virus. Molecular and Cellular Probes, 20, 203-211.
PMID |
| [22] | Hou L, Xue B, Xue HY (2019) Study on soil nematode community of Northern Tibetan alpine meadow under simulated warming condition by high-throughput sequencing method. Acta Agrestia Sinica, 27, 443-451. (in Chinese with English abstract) |
|
[ 侯磊, 薛蓓, 薛会英 (2019) 利用高通量测序法对模拟增温条件下藏北高寒草甸土壤线虫群落的研究. 草地学报, 27, 443-451.]
DOI |
|
| [23] |
Kawanobe M, Toyota K, Ritz K (2021) Development and application of a DNA metabarcoding method for comprehensive analysis of soil nematode communities. Applied Soil Ecology, 166, 103974.
DOI URL |
| [24] | Kenmotsu H, Takabayashi E, Takase A, Hirose Y, Eki T(2021) Use of universal primers for the 18S ribosomal RNA gene and whole soil DNAs to reveal the taxonomic structures of soil nematodes by high-throughput amplicon sequencing. PLoS ONE, 16, e0259842. |
| [25] | Kitagami Y, Obase K, Matsuda Y (2022) High-throughput sequencing and conventional morphotyping show different soil nematode assemblages but similar community responses to altitudinal gradients on Mt. Ibuki, Japan. Pedobiologia, 90, 150788. |
| [26] |
Kushida A (2013) Design and evaluation of PCR primers for denaturing gradient gel electrophoresis analysis of plant parasitic and fungivorous nematode communities. Microbes and Environments, 28, 269-274.
DOI PMID |
| [27] |
Li B, Li YB, Fanin N, Han X, Du XF, Liu HW, Li YH, Li Q (2022) Adaptation of soil micro-food web to elemental limitation: Evidence from the forest-steppe ecotone. Soil Biology and Biochemistry, 170, 108698.
DOI URL |
| [28] | Li Q, Liang WJ, Zhang XK, Mahamood M (2017) Soil Nematodes of Grasslands in Northern China. Zhejiang University Press, Hangzhou & Elsevier Academic Press, Amsterdam. |
| [29] |
Li YB, Liang SW, Du XF, Kou XC, Lv XT, Li Q (2021) Mowing did not mitigate the negative effects of nitrogen deposition on soil nematode community in a temperate steppe. Soil Ecology Letters, 3, 125-133.
DOI |
| [30] |
Liu HW, Du XF, Li YB, Han X, Li B, Zhang XK, Li Q, Liang WJ (2022) Organic substitutions improve soil quality and maize yield through increasing soil microbial diversity. Journal of Cleaner Production, 347, 131323.
DOI URL |
| [31] |
Lott MJ, Hose GC, Power ML (2014) Towards the molecular characterisation of parasitic nematode assemblages: An evaluation of terminal-restriction fragment length polymorphism (T-RFLP) analysis. Experimental Parasitology, 144, 76-83.
DOI PMID |
| [32] |
Madani M, Subbotin SA, Moens M (2005) Quantitative detection of the potato cyst nematode, Globodera pallida, and the beet cyst nematode, Heterodera schachtii, using real-time PCR with SYBR green I dye. Molecular and Cellular Probes, 19, 81-86.
DOI URL |
| [33] |
Muyzer G, Dewaal EC, Uitterlinden AG (1993) Profiling of complex microbial populations by denaturing gradient gel electrophoresis analysis of polymerase chain reaction- amplified genes coding for 16S rRNA. Applied and Environmental Microbiology, 59, 695-700.
DOI PMID |
| [34] |
Nakhla MK, Owens KJ, Li WB, Wei G, Skantar AM, Levy L (2010) Multiplex real-time PCR assays for the identification of the potato cyst and tobacco cyst nematodes. Plant Disease, 94, 959-965.
DOI PMID |
| [35] |
Nisa RU, Tantray AY, Shah AA (2022) Shift from morphological to recent advanced molecular approaches for the identification of nematodes. Genomics, 114, 110295.
DOI URL |
| [36] |
Ovreås L, Forney L, Daae FL, Torsvik V (1997) Distribution of bacterioplankton in meromictic lake Saelenvannet, as determined by denaturing gradient gel electrophoresis of PCR-amplified gene fragments coding for 16S rRNA. Applied and Environmental Microbiology, 63, 3367-3373.
DOI PMID |
| [37] | Peham T, Steiner FM, Schlick-Steiner BC, Arthofer W (2017) Are we ready to detect nematode diversity by next generation sequencing? Ecology and Evolution, 7, 4147-4151. |
| [38] |
Powers T (2004) Nematode molecular diagnostics: From bands to barcodes. Annual Review of Phytopathology, 42, 367-383.
PMID |
| [39] |
Rettedal EA, Clay S, Brözel VS (2010) GC-clamp primer batches yield 16S rRNA gene amplicon pools with variable GC clamps, affecting denaturing gradient gel electrophoresis profiles. FEMS Microbiology Letters, 312, 55-62.
DOI PMID |
| [40] |
Sapkota R, Nicolaisen M (2015) High-throughput sequencing of nematode communities from total soil DNA extractions. BMC Ecology, 15, 3.
DOI PMID |
| [41] | Schenk J, Geisen S, Kleinbölting N, Traunspurger W (2019) Metabarcoding data allow for reliable biomass estimates in the most abundant animals on earth. Metabarcoding and Metagenomics, 3, e46704. |
| [42] |
Schenk J, Kleinbölting N, Traunspurger W (2020) Comparison of morphological, DNA barcoding, and metabarcoding characterizations of freshwater nematode communities. Ecology and Evolution, 10, 2885-2899.
DOI PMID |
| [43] |
Sikder MM, Vestergård M, Sapkota R, Kyndt T, Nicolaisen M (2020) Evaluation of metabarcoding primers for analysis of soil nematode communities. Diversity, 12, 388.
DOI URL |
| [44] |
Sun YX, Du XF, Li YB, Han X, Fang S, Geisen S, Li Q (2023) Database and primer selections affect nematode community composition under different vegetations of Changbai Mountain. Soil Ecology Letters, 5, 142-150.
DOI |
| [45] |
Tedersoo L, Drenkhan R, Anslan S, Morales-Rodriguez C, Cleary M (2019) High-throughput identification and diagnostics of pathogens and pests: Overview and practical recommendations. Molecular Ecology Resources, 19, 47-76.
DOI PMID |
| [46] |
Treonis AM, Unangst SK, Kepler RM, Buyer JS, Cavigelli MA, Mirsky SB, Maul JE (2018) Characterization of soil nematode communities in three cropping systems through morphological and DNA metabarcoding approaches. Scientific Reports, 8, 2004.
DOI PMID |
| [47] |
Waeyenberge L, de Sutter N, Viaene N, Haegeman A (2019) New insights into nematode DNA-metabarcoding as revealed by the characterization of artificial and spiked nematode communities. Diversity, 11, 1-22.
DOI URL |
| [48] | Wang IC (2012) Developing a qPCR-based Molecular Technique for Nematode Community Analysis. PhD dissertation, University of Hawaii, Hawaii. |
| [49] |
Wang N, Huang JH, Huo N, Yang PP, Zhang XY, Zhao SW (2021) Characteristics of soil nematode community under different vegetation restoration approaches in the mountainous region of southern Ningxia: A comparative study based on morphological identification and high-throughput sequencing methods. Biodiversity Science, 29, 1513-1529. (in Chinese with English abstract)
DOI |
|
[ 王楠, 黄菁华, 霍娜, 杨盼盼, 张欣玥, 赵世伟 (2021) 宁南山区不同植被恢复方式下土壤线虫群落特征: 形态学鉴定与高通量测序法比较. 生物多样性, 29, 1513-1529.]
DOI |
|
| [50] |
Wang SB, Li Q, Liang WJ, Jiang Y, Jiang SW (2008) PCR- DGGE analysis of nematode diversity in Cu-contaminated soil. Pedosphere, 18, 621-627.
DOI URL |
| [51] |
Wiesel L, Daniell TJ, King D, Neilson R (2015) Determination of the optimal soil sample size to accurately characterise nematode communities in soil. Soil Biology and Biochemistry, 80, 89-91.
DOI URL |
| [1] | 罗正明, 刘晋仙, 张变华, 周妍英, 郝爱华, 杨凯, 柴宝峰. 不同退化阶段亚高山草甸土壤原生生物群落多样性特征及驱动因素[J]. 生物多样性, 2023, 31(8): 23136-. |
| [2] | 毛莹儿, 周秀梅, 王楠, 李秀秀, 尤育克, 白尚斌. 毛竹扩张对杉木林土壤细菌群落的影响[J]. 生物多样性, 2023, 31(6): 22659-. |
| [3] | 赵雯, 王丹丹, 热依拉·木民, 黄开钏, 刘顺, 崔宝凯. 阿尔山地区兴安落叶松林土壤微生物群落结构[J]. 生物多样性, 2023, 31(2): 22258-. |
| [4] | 夏凡, 杨婧, 李建, 史洋, 盖立新, 黄文华, 张经纬, 杨南, 高福利, 韩莹莹, 鲍伟东. 北京地区四个豹猫亚种群肠道菌群的组成[J]. 生物多样性, 2022, 30(9): 22103-. |
| [5] | 刘笑彤, 田艺佳, 刘汉文, 梁翠影, 姜思维, 梁文举, 张晓珂. 下辽河平原农田土壤线虫群落组成的季节变化[J]. 生物多样性, 2022, 30(12): 22222-. |
| [6] | 高程, 郭良栋. 微生物物种多样性、群落构建与功能性状研究进展[J]. 生物多样性, 2022, 30(10): 22429-. |
| [7] | 夏呈强, 李毅, 党延茹, 察倩倩, 贺晓艳, 秦启龙. 中印度洋与南海西部表层海水细菌多样性[J]. 生物多样性, 2022, 30(1): 21407-. |
| [8] | 陆奇丰, 黄至欢, 骆文华. 极小种群濒危植物广西火桐、丹霞梧桐的叶绿体基因组特征[J]. 生物多样性, 2021, 29(5): 586-595. |
| [9] | 王楠, 黄菁华, 霍娜, 杨盼盼, 张欣玥, 赵世伟. 宁南山区不同植被恢复方式下土壤线虫群落特征:形态学鉴定与高通量测序法比较[J]. 生物多样性, 2021, 29(11): 1513-1529. |
| [10] | 靳新影, 张肖冲, 金多, 陈韵, 李靖宇. 腾格里沙漠东南缘不同生物土壤结皮细菌多样性及其季节动态特征[J]. 生物多样性, 2020, 28(6): 718-726. |
| [11] | 韩本凤, 周欣, 张雪. 基因组学技术在病毒鉴定与宿主溯源中的应用[J]. 生物多样性, 2020, 28(5): 587-595. |
| [12] | 张全建, 杨彪, 付强, 王磊, 龚旭, 张远彬. 邛崃山系水鹿的冬季食性[J]. 生物多样性, 2020, 28(10): 1192-1201. |
| [13] | 陆琪,胡强,施小刚,金森龙,李晟,姚蒙. 基于分子宏条形码分析四川卧龙国家级自然保护区雪豹的食性[J]. 生物多样性, 2019, 27(9): 960-969. |
| [14] | 刘君, 王宁, 崔岱宗, 卢磊, 赵敏. 小兴安岭大亮子河国家森林公园不同生境下土壤细菌多样性和群落结构[J]. 生物多样性, 2019, 27(8): 911-918. |
| [15] | 刘山林. DNA条形码参考数据集构建和序列分析相关的新兴技术[J]. 生物多样性, 2019, 27(5): 526-533. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
![]()