



Biodiv Sci ›› 2017, Vol. 25 ›› Issue (12): 1276-1284. DOI: 10.17520/biods.2017301 cstr: 32101.14.biods.2017301
Special Issue: 生物入侵
• Special Feature: Biological Invasion • Previous Articles Next Articles
Fang Zhou, Zhijie Zhang, Mu Liu, Xiaoyun Pan*( )
)
Received:2017-11-09
Accepted:2017-12-26
Online:2017-12-20
Published:2017-12-10
Contact:
Pan Xiaoyun
Fang Zhou, Zhijie Zhang, Mu Liu, Xiaoyun Pan. Effects of nutrient levels on defense against specialist insects in an invasive alligator weed[J]. Biodiv Sci, 2017, 25(12): 1276-1284.
| 参数 Source of variation | d.f | F | |||
|---|---|---|---|---|---|
| 总生物量 Total biomass | 贮藏根生物量 SRB | 根冠比 RSR | 比茎长 SSL | ||
| 采样地 Origin (O) | 1, 16 | 36.76*** | 2.07 | 3.67 | 51.71*** |
| 种群 Population (Pop) | 16, 264 | 9.01*** | 5.69*** | 10.05*** | 14.03*** |
| 养分 Nutrient (N) | 1, 264 | 1.56 | 53.41*** | 126.82*** | 57.00*** |
| 取食 Herbivory (H) | 1, 264 | 16.84*** | 7.23** | 10.46** | 3.86 |
| 养分 × 取食 (N × H) | 1, 264 | 37.81*** | 25.75*** | 46.80*** | 0.95 |
| 采样地 × 养分 (O × N) | 1, 264 | 0.33 | 0.74 | 0.10 | 0.36 |
| 采样地 × 取食 (O × H) | 1, 264 | 2.14 | 0.05 | 0.09 | 0.12 |
| 采样地 × 养分 × 取食 (O × N × H) | 1, 264 | 0.40 | 0.01 | 0.68 | 5.15* |
| 养分 × 种群 (N × Pop) | 16, 264 | 1.69 | 0.57 | 1.49 | 1.24 |
| 养分 × 取食 × 种群 (N × H × Pop) | 16, 264 | 1.51 | 0.95 | 1.49 | 1.37 |
| 协变量 Covariates | 1, 287 | 45.18*** | 2.15 | 23.74*** | |
Table 1 ANOVA or ANCOVA of the effects of continental origin, population (pop), nutrient level and herbivory on total biomass, storage root biomass (SRB), root/shoot ratio (RSR), specific stem length (SSL) of Alternanthera philoxeroides.
| 参数 Source of variation | d.f | F | |||
|---|---|---|---|---|---|
| 总生物量 Total biomass | 贮藏根生物量 SRB | 根冠比 RSR | 比茎长 SSL | ||
| 采样地 Origin (O) | 1, 16 | 36.76*** | 2.07 | 3.67 | 51.71*** |
| 种群 Population (Pop) | 16, 264 | 9.01*** | 5.69*** | 10.05*** | 14.03*** |
| 养分 Nutrient (N) | 1, 264 | 1.56 | 53.41*** | 126.82*** | 57.00*** |
| 取食 Herbivory (H) | 1, 264 | 16.84*** | 7.23** | 10.46** | 3.86 |
| 养分 × 取食 (N × H) | 1, 264 | 37.81*** | 25.75*** | 46.80*** | 0.95 |
| 采样地 × 养分 (O × N) | 1, 264 | 0.33 | 0.74 | 0.10 | 0.36 |
| 采样地 × 取食 (O × H) | 1, 264 | 2.14 | 0.05 | 0.09 | 0.12 |
| 采样地 × 养分 × 取食 (O × N × H) | 1, 264 | 0.40 | 0.01 | 0.68 | 5.15* |
| 养分 × 种群 (N × Pop) | 16, 264 | 1.69 | 0.57 | 1.49 | 1.24 |
| 养分 × 取食 × 种群 (N × H × Pop) | 16, 264 | 1.51 | 0.95 | 1.49 | 1.37 |
| 协变量 Covariates | 1, 287 | 45.18*** | 2.15 | 23.74*** | |
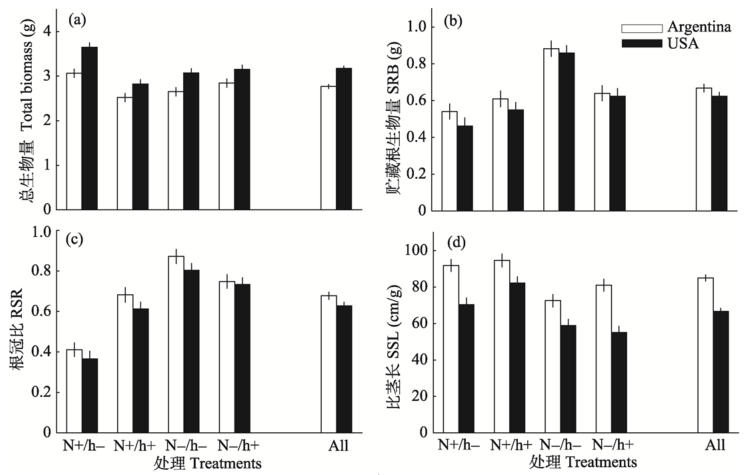
Fig. 1 The effects of herbivory by Agasicles hygrophila (with, h+, without, h-) and nutrient level (high, N+, low, N-) on plant total biomass (a), storage root biomass (SRB) (b), root/shoot ratio (RSR) (c), and specific stem length (SSL) (d) of Alternanthera philoxeroides from native (Argentina) and introduced (USA) range. Error bars denote +/- SE.
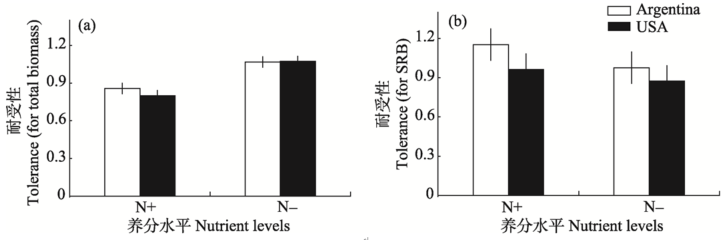
Fig. 2 The effects of nutrient level (high, N+, low, N-) on tolerance of total biomass (a) and SRB (b) to herbivory by Agasicles hygrophila of Alternanthera philoxeroides from native (Argentina) and introduced (USA) range. Error bars denote +/- SE.
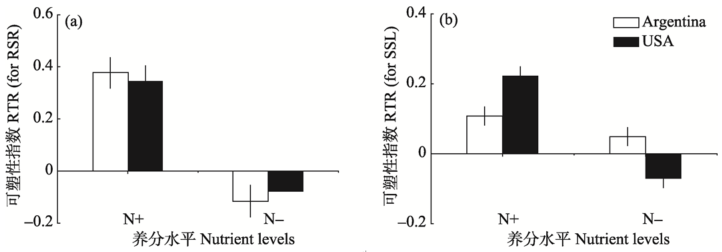
Fig. 3 The effects of nutrient level (high, N+, low, N-) on the plasticity (RTR) of RSR (a) and SSL (b) in response to herbivory by Agasicles hygrophila of Alternanthera philoxeroides from native (Argentina) and introduced (USA) range. Error bars denote +/-SE.
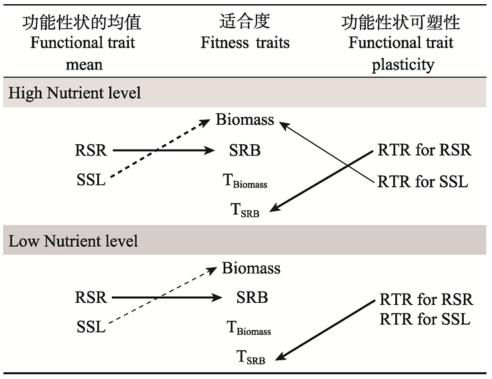
Fig. 4 The correlations between plant fitness (total biomass, SRB), tolerance of total biomass (TBiomass) and SRB (TSRB) to herbivory and functional traits (root/shoot ratio, RSR; specific stem length, SSL), relative trait range of functional traits (RTR for RSR, RTR for SSL) in response to herbivory by Agasicles hygrophila for Alternanthera philoxeroides from native (Argentina) and introduced (USA) range grown under high and low nutrient levels. The solid lines signify positive correlation, and dashed ones mean negative, the thick lines mean significant at P < 0.01, and thin lines at P < 0.05.
| [1] | Abhilasha D, Joshi J (2009) Enhanced fitness due to higher fecundity, increased defence against a specialist and toler¬ance towards a generalist herbivore in an invasive annual plant. Journal of Plant Ecology, 2, 77-86. |
| [2] | Agrawal AA, Hastings AP, Bradburd GS, Woods EC, Zust T, Harvey JA, Bukovinszky T (2015) Evolution of plant growth and defense in a continental introduction. The American Naturalist, 186, E1-E15. |
| [3] | Beaton LL, Van Zandt PA, Esselman EJ, Knight TM (2011) Comparison of the herbivore defense and competitive ability of ancestral and modern genotypes of an invasive plant, Lespedeza cuneata. Oikos, 120, 1413-1419. |
| [4] | Bjorkman C, Bommarco R, Eklund K, Hoglund S (2004) Harvesting disrupts biological control of herbivores in a short-rotation coppice system. Ecological Applications, 14, 1624-1633. |
| [5] | Blossey B, Notzold R (1995) Evolution of increased compe¬titive ability in invasive nonindigenous plants—a hypothesis. Journal of Ecology, 83, 887-889. |
| [6] | Bossdorf O, Schroder S, Prati D, Auge H (2004) Palatability and tolerance to simulated herbivory in native and introduced populations of Alliaria petiolata (Brassicaceae). American Journal of Botany, 91, 856-862. |
| [7] | Burghardt KT (2016) Nutrient supply alters goldenrod’s induced response to herbivory. Functional Ecology, 30, 1769-1778. |
| [8] | Cano L, Escarre J, Fleck I, Blanco-Moreno JM, Sans FX (2008) Increased fitness and plasticity of an invasive species in its introduced range: a study using Senecio pterophorus. Journal of Ecology, 96, 468-476. |
| [9] | Cipollini D, Mbagwu J, Barto K, Hillstrom C, Enright S (2005) Expression of constitutive and inducible chemical defenses in native and invasive populations of Alliaria petiolata. Journal of Chemical Ecology, 31, 1255-1267. |
| [10] | Colautti RI, Maron JL, Barrett SCH (2009) Common garden comparisons of native and introduced plant populations: latitudinal clines can obscure evolutionary inferences. Evolutionary Applications, 2, 187-199. |
| [11] | Coley PD, Bryant JP, Chapin FS (1985) Resource availability and plant antiherbivore defense. Science, 230, 895-899. |
| [12] | Davis MA, Grime JP, Thompson K (2000) Fluctuating resou¬rces in plant communities: a general theory of invasibility. Journal of Ecology, 88, 528-534. |
| [13] | Endara MJ, Coley PD (2011) The resource availability hypot¬hesis revisited: a meta-analysis. Functional Ecology, 25, 389-398. |
| [14] | Fornoni J (2011) Ecological and evolutionary implications of plant tolerance to herbivory. Functional Ecology, 25, 399-407. |
| [15] | Genton BJ, Kotanen PM, Cheptou PO, Adolphe C, Shykoff JA (2005) Enemy release but no evolutionary loss of defence in a plant invasion: an inter-continental reciprocal transplant experiment. Oecologia, 146, 404-414. |
| [16] | Huang W, Carrillo J, Ding JQ, Siemann E (2012) Invader partitions ecological and evolutionary responses to above- and belowground herbivory. Ecology, 93, 2343-2352. |
| [17] | Huang W, Siemann E, Wheeler GS, Zou JW, Carrillo J, Ding JQ (2010) Resource allocation to defence and growth are driven by different responses to generalist and specialist herbivory in an invasive plant. Journal of Ecology, 98, 1157-1167. |
| [18] | Julien MH, Broadbent JE (1980) The biology of Australian weeds. 3. Alternanthera philoxeroides (Mart.) Griseb. Journal of the Australian Institute of Agricultural Science, 46, 150-155. |
| [19] | Julien MH, Skarratt B, Maywald GF (1995) Potential geographical distribution of alligator weed and its biological control by Agasicles hygrophila. Journal of Aquatic Plant Management, 33, 55-60. |
| [20] | Keane RM, Crawley MJ (2002) Exotic plant invasions and the enemy release hypothesis. Trends in Ecology & Evolution, 17, 164-170. |
| [21] | Koricheva J, Nykanen H, Gianoli E (2004) Meta-analysis of trade-offs among plant antiherbivore defenses: are plants jacks-of-all-trades, masters of all? The American Naturalist, 163, E64-E75. |
| [22] | Kumschick S, Hufbauer RA, Alba C, Blumenthal DM (2013) Evolution of fast-growing and more resistant phenotypes in introduced common mullein (Verbascum thapsus). Journal of Ecology, 101, 378-387. |
| [23] | Li YP, Feng YL, Barclay G (2012) No evidence for evolutio¬narily decreased tolerance and increased fitness in invasive Chromolaena odorata: implications for invasi¬veness and biological control. Plant Ecology, 213, 1157-1166. |
| [24] | Lu XM, Siemann E, He MY, Wei H, Shao X, Ding JQ (2015) Climate warming increases biological control agent impact on a non-target species. Ecology Letters, 18, 48-56. |
| [25] | Lu XM, Siemann E, Shao X, Wei H, Ding JQ (2013) Climate warming affects biological invasions by shifting interactions of plants and herbivores. Global Change Biology, 19, 2339-2347. |
| [26] | Maddox DM, Andres LA, Hennessey RD, Blackburn RD, Spe¬ncer NR (1971) Insects to control alligator weed: an invader of aquatic ecosystems in the United States. BioScience, 21, 985-991. |
| [27] | Pan XY, Geng YP, Li B, Chen JK (2006) Phenotypic plasticity and dominance shift of co-occurring native and alien inva¬sive in contrasting habitats. Acta Oecologica, 30, 333-341. |
| [28] | Pan XY, Geng YP, Sosa A, Zhang WJ, Li B, Chen JK (2007) Invasive Alternanthera philoxeroides: biology, ecology and management. Acta Phytotaxonomica Sinica, 45, 884-900. |
| [29] | Pan XY, Jia X, Fu DJ, Li B (2013) Geographical diversification of growth-defense strategies in an invasive plant. Journal of Systematics and Evolution, 51, 308-317. |
| [30] | Qing H, Yao YH, Xiao Y, Hu FQ, Sun YX, Zhou CF, An SQ (2011) Invasive and native tall forms of Spartina alterniflo¬ra respond differently to nitrogen availability. Acta Oecol¬og¬ica, 37, 23-30. |
| [31] | Quiroz CL, Choler P, Baptist F, Gonzalez-Teuber M, Moli¬na-Montenegro MA, Cavieres LA (2009) Alpine dandelions originated in the native and introduced range differ in their responses to environmental constraints. Ecological Resea¬rch, 24, 175-183. |
| [32] | Richards CL, Bossdorf O, Muth NZ, Gurevitch J, Pigliucci M (2006) Jack of all trades, master of some? On the role of phenotypic plasticity in plant invasions. Ecology Letters, 9, 981-993. |
| [33] | Rogers WE, Siemann E (2004) Invasive ecotypes tolerate herbivory more effectively than native ecotypes of the Chinese tallow tree Sapium sebiferum. Journal of Applied Ecology, 41, 561-570. |
| [34] | Stout MJ, Brovont RA, Duffey SS (1998) Effect of nitrogen availability on expression of constitutive and inducible chemical defenses in tomato, Lycopersicon esculentum. Journal of Chemical Ecology, 24, 945-963. |
| [35] | Strauss SY, Agrawal AA (1999) The ecology and evolution of plant tolerance to herbivory. Trends in Ecology & Evol¬ution, 14, 179-185. |
| [36] | Sun Y, Ding JQ, Rena MX (2009) Effects of simulated herb¬ivory and resource availability on the invasive plant, Alter¬nanthera philoxeroides in different habitats. Biological Con¬trol, 48, 287-293. |
| [37] | Wei H, Lu XM, Ding JQ (2015) Direct and indirect impacts of different water regimes on the invasive plant, alligator weed (Alternanthera philoxeroides), and its biological control agent, Agasicles hygrophila. Weed Biology and Managem¬ent, 15, 1-10. |
| [38] | Wise MJ, Abrahamson WG (2007) Effects of resource avail¬ability on tolerance of herbivory: a review and assessment of three opposing models. The American Naturalist, 169, 443-454. |
| [39] | Zhang HJ, Chang RY, Guo X, Liang XQ, Wang RQ, Liu J (2017) Shifts in growth and competitive dominance of the invasive plant Alternanthera philoxeroides under different nitrogen and phosphorus supply. Environmental and Experimental Botany, 135, 118-125. |
| [40] | Zhang ZJ, Pan XY, Zhang ZY, He KS, Li B (2015) Specialist insect herbivore and light availability do not interact in the evolution of an invasive plant. PLoS ONE, 10, 14. |
| [41] | Zou JW, Siemann E, Rogers WE, DeWalt SJ (2008) Decreased resistance and increased tolerance to native herbivores of the invasive plant Sapium sebiferum. Ecography, 31, 663-671. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn ![]()