



生物多样性 ›› 2023, Vol. 31 ›› Issue (3): 22600. DOI: 10.17520/biods.2022600 cstr: 32101.14.biods.2022600
所属专题: 土壤生物与土壤健康
• 研究报告: 植物多样性 • 上一篇
吴浩1,2, 余玉蓉1,2, 王佳钰1,2, 赵媛博1,2, 高娅菲1,2, 李小玲2,3, 卜贵军1,2, 薛丹4, 吴林1,2,*( )
)
收稿日期:2022-10-24
接受日期:2023-01-16
出版日期:2023-03-20
发布日期:2023-03-20
通讯作者:
吴林
作者简介:* E-mail: wulin2019@yeah.net基金资助:
Hao Wu1,2, Yurong Yu1,2, Jiayu Wang1,2, Yuanbo Zhao1,2, Yafei Gao1,2, Xiaoling Li2,3, Guijun Bu1,2, Dan Xue4, Lin Wu1,2,*( )
)
Received:2022-10-24
Accepted:2023-01-16
Online:2023-03-20
Published:2023-03-20
Contact:
Lin Wu
摘要:
地下水位变化对泥炭地的植被组成及多样性具有明显的调控作用, 从而可能会深刻改变泥炭地的储碳潜力。目前, 有关泥炭地植物多样性和土壤有机碳含量对水位波动的响应还存在较大争议, 且有关亚热带贫营养泥炭地地下水位对植物多样性及生物量与土壤有机碳含量影响的研究鲜有报道。本研究选择鄂西南贫营养泥炭地为研究对象, 调查了4个地下水位梯度(-4 cm、-8 cm、-12 cm、-20 cm)下的植被组成、多样性、生物量及土壤有机碳含量, 以探究不同水位梯度对鄂西南贫营养泥炭地植物多样性、生物量及土壤有机碳含量的影响。结果表明: (1)地下水位下降, 土壤含水量、土壤有机碳含量和总酚含量显著降低, 而溶解氧含量显著增加(P < 0.05)。并且, 低水位(-20 cm)处土壤有机碳含量是高水位(-4 cm)处土壤有机碳含量的72%。(2)地下水位显著改变鄂西南贫营养泥炭地物种组成, 随着地下水位下降, 灌木物种数量增加, 且以浅根系的杜鹃花科和蔷薇科植物为主。(3)总体上, 随着地下水位的降低, 灌木多样性呈现显著增加的趋势(P < 0.05), 而草本植物多样性变化不显著。(4)地下水位对植被地上总体生物量影响不显著, 但随地下水位的降低, 灌木生物量极显著增加(P < 0.01)、草本生物量显著增加(P < 0.05), 而苔藓生物量降低。本研究表明, 较高的地下水位是维持鄂西南贫营养泥炭地土壤有机碳含量的关键, 维管植物多样性的提升并不能增加该泥炭地的固碳潜力。
吴浩, 余玉蓉, 王佳钰, 赵媛博, 高娅菲, 李小玲, 卜贵军, 薛丹, 吴林 (2023) 低水位增加灌木多样性和生物量但降低土壤有机碳含量: 以鄂西南贫营养泥炭地为例. 生物多样性, 31, 22600. DOI: 10.17520/biods.2022600.
Hao Wu, Yurong Yu, Jiayu Wang, Yuanbo Zhao, Yafei Gao, Xiaoling Li, Guijun Bu, Dan Xue, Lin Wu (2023) Lower water table increase shrub plant diversity and biomass but decrease soil organic carbon content: A case study of oligotrophic peatland in the Southwestern Hubei Province. Biodiversity Science, 31, 22600. DOI: 10.17520/biods.2022600.
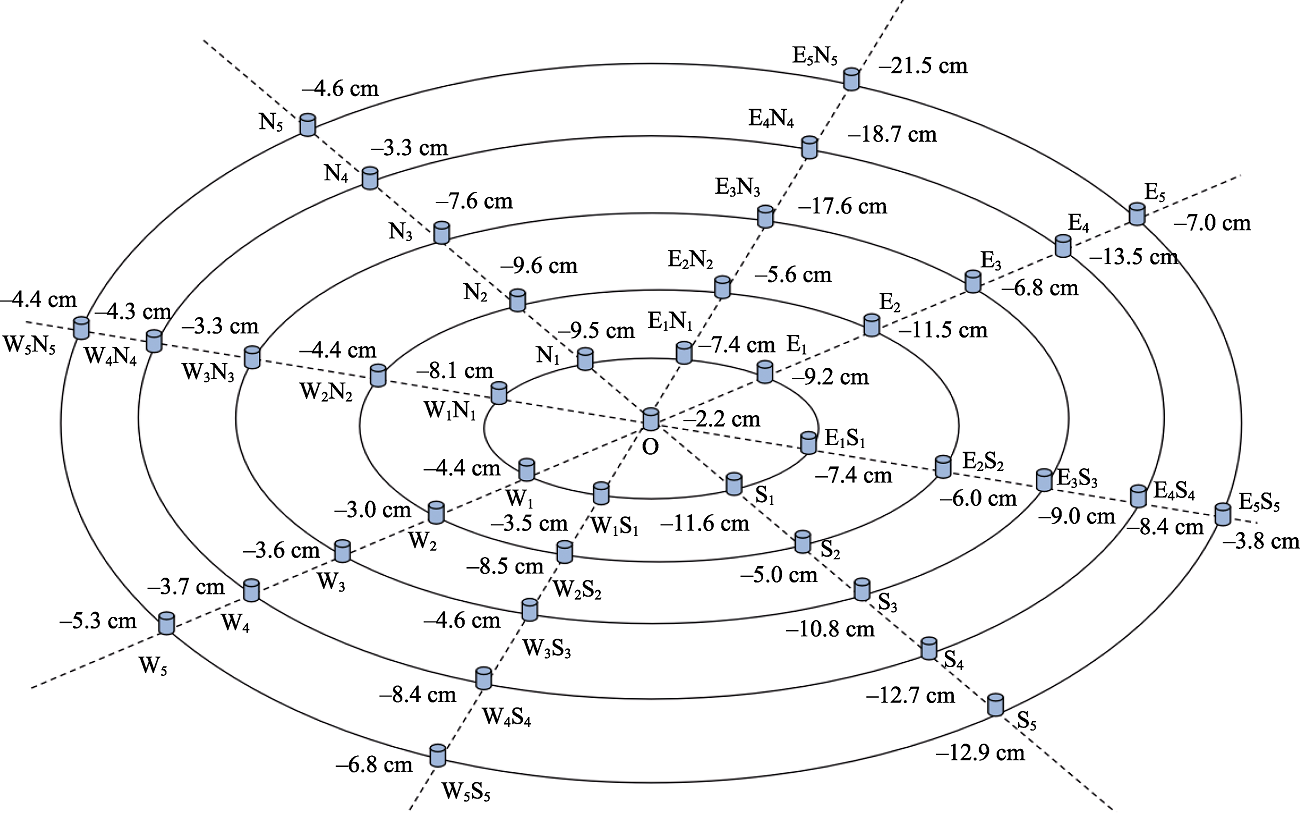
图1 鄂西南贫营养泥炭地地下水位监测点分布图及其地下水位深度(cm)
Fig. 1 Distribution map and depth of water table level (cm) in oligotrophic peatland in the Southwestern Hubei Province
| 水位 Water table (cm) | 土壤pH Soil pH | 土壤容重 Soil bulk density (g/cm) | 土壤含水量 Soil water content (%) | 溶解氧 Dissolved oxygen (mg/L) | 总酚 Total phenols (μg/mL) | 土壤有机碳 Soil organic carbon (%) |
|---|---|---|---|---|---|---|
| -4 | 3.97 ± 0.15a | 0.20 ± 0.01a | 83.51 ± 0.79c | 5.27 ± 0.48a | 6.23 ± 0.37b | 33.88 ± 2.17b |
| -8 | 4.18 ± 0.05a | 0.22 ± 0.01a | 81.57 ± 1.22bc | 5.72 ± 0.42a | 6.01 ± 1.38b | 33.20 ± 0.92b |
| -12 | 4.22 ± 0.17a | 0.29 ± 0.03b | 78.10 ± 1.74ab | 7.23 ± 0.18b | 3.99 ± 0.58ab | 23.58 ± 1.12a |
| -20 | 4.40 ± 0.18a | 0.33 ± 0.02b | 74.17 ± 0.87a | 7.13 ± 0.23b | 2.80 ± 0.55a | 24.31 ± 2.36a |
表1 鄂西南不同地下水位梯度下贫营养泥炭地土壤理化性质
Table 1 Soil physicochemical properties under different water table level gradients in a oligotrophic peatland in the Southwestern Hubei Province
| 水位 Water table (cm) | 土壤pH Soil pH | 土壤容重 Soil bulk density (g/cm) | 土壤含水量 Soil water content (%) | 溶解氧 Dissolved oxygen (mg/L) | 总酚 Total phenols (μg/mL) | 土壤有机碳 Soil organic carbon (%) |
|---|---|---|---|---|---|---|
| -4 | 3.97 ± 0.15a | 0.20 ± 0.01a | 83.51 ± 0.79c | 5.27 ± 0.48a | 6.23 ± 0.37b | 33.88 ± 2.17b |
| -8 | 4.18 ± 0.05a | 0.22 ± 0.01a | 81.57 ± 1.22bc | 5.72 ± 0.42a | 6.01 ± 1.38b | 33.20 ± 0.92b |
| -12 | 4.22 ± 0.17a | 0.29 ± 0.03b | 78.10 ± 1.74ab | 7.23 ± 0.18b | 3.99 ± 0.58ab | 23.58 ± 1.12a |
| -20 | 4.40 ± 0.18a | 0.33 ± 0.02b | 74.17 ± 0.87a | 7.13 ± 0.23b | 2.80 ± 0.55a | 24.31 ± 2.36a |
| 科 Family | 水位 Water table | 全部Total | |||
|---|---|---|---|---|---|
| -4 cm | -8 cm | -12 cm | -20 cm | ||
| 蔷薇科 Rosaceae | 1 | 1 | 1 | 4 | 4 |
| 杜鹃花科 Ericaceae | 1 | 3 | 3 | 3 | 3 |
| 樟科 Lauraceae | - | 1 | 1 | - | 1 |
| 槭树科 Aceraceae | - | 1 | 1 | - | 1 |
| 壳斗科 Fagaceae | - | 1 | 1 | 1 | 1 |
| 大戟科 Euphorbiaceae | - | - | 1 | - | 1 |
| 冬青科 Aquifoliaceae | 1 | 1 | 1 | 1 | 1 |
| 虎耳草科 Saxifragaceae | 1 | - | 1 | 1 | 1 |
| 三尖杉科 Cephalotaxaceae | - | - | 1 | - | 1 |
| 金丝桃科 Hypericaceae | 1 | - | 1 | - | 1 |
| 忍冬科 Caprifoliaceae | - | - | 2 | 1 | 2 |
| 柏科 Cupressaceae | - | - | 1 | - | 1 |
| 莎草科 Cyperaceae | 1 | 1 | 1 | - | 1 |
| 碗蕨科 Dennstaedtiaceae | - | - | 1 | 1 | 1 |
| 石松科 Lycopodiaceae | - | - | - | 1 | 1 |
| 禾本科 Gramineae | 1 | 1 | - | 1 | 2 |
| 金星蕨科 Thelypteridaceae | - | 1 | 1 | - | 1 |
| 百合科 Liliaceae | - | - | 1 | - | 1 |
| 灯芯草科 Juncaceae | 1 | - | - | 1 | 1 |
| 龙胆科 Gentianaceae | 1 | - | - | - | 1 |
| 泥炭藓科 Sphagnaceae | 1 | 1 | 1 | 1 | 1 |
| 金发藓科 Polytrichaceae | - | - | 1 | 1 | 2 |
| 合计 Total | 10 | 12 | 21 | 17 | 30 |
表2 鄂西南不同地下水位梯度下贫营养泥炭地物种数
Table 2 No. of species composition under different water table level gradients in oligotrophic peatland in the Southwestern Hubei Province
| 科 Family | 水位 Water table | 全部Total | |||
|---|---|---|---|---|---|
| -4 cm | -8 cm | -12 cm | -20 cm | ||
| 蔷薇科 Rosaceae | 1 | 1 | 1 | 4 | 4 |
| 杜鹃花科 Ericaceae | 1 | 3 | 3 | 3 | 3 |
| 樟科 Lauraceae | - | 1 | 1 | - | 1 |
| 槭树科 Aceraceae | - | 1 | 1 | - | 1 |
| 壳斗科 Fagaceae | - | 1 | 1 | 1 | 1 |
| 大戟科 Euphorbiaceae | - | - | 1 | - | 1 |
| 冬青科 Aquifoliaceae | 1 | 1 | 1 | 1 | 1 |
| 虎耳草科 Saxifragaceae | 1 | - | 1 | 1 | 1 |
| 三尖杉科 Cephalotaxaceae | - | - | 1 | - | 1 |
| 金丝桃科 Hypericaceae | 1 | - | 1 | - | 1 |
| 忍冬科 Caprifoliaceae | - | - | 2 | 1 | 2 |
| 柏科 Cupressaceae | - | - | 1 | - | 1 |
| 莎草科 Cyperaceae | 1 | 1 | 1 | - | 1 |
| 碗蕨科 Dennstaedtiaceae | - | - | 1 | 1 | 1 |
| 石松科 Lycopodiaceae | - | - | - | 1 | 1 |
| 禾本科 Gramineae | 1 | 1 | - | 1 | 2 |
| 金星蕨科 Thelypteridaceae | - | 1 | 1 | - | 1 |
| 百合科 Liliaceae | - | - | 1 | - | 1 |
| 灯芯草科 Juncaceae | 1 | - | - | 1 | 1 |
| 龙胆科 Gentianaceae | 1 | - | - | - | 1 |
| 泥炭藓科 Sphagnaceae | 1 | 1 | 1 | 1 | 1 |
| 金发藓科 Polytrichaceae | - | - | 1 | 1 | 2 |
| 合计 Total | 10 | 12 | 21 | 17 | 30 |
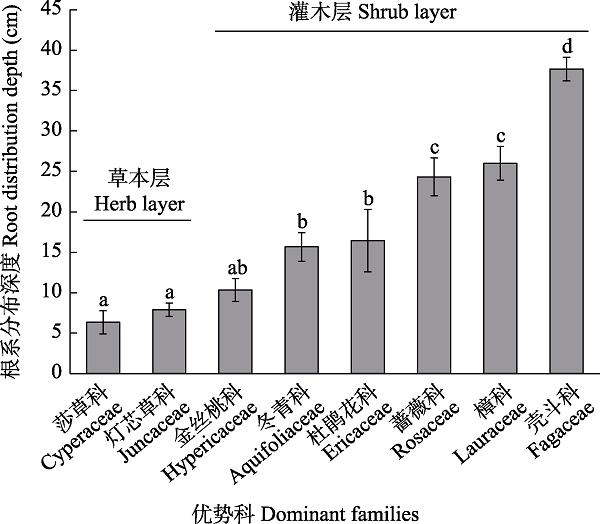
图2 鄂西南贫营养泥炭地不同优势科植物根系分布深度(平均值 ± 标准误)。不同小写字母表示不同科之间存在显著差异(P < 0.05)。
Fig. 2 Root distribution depth of different dominant families of plants in oligotrophic peatland in the Southwestern Hubei Province (mean ± SE). Different lowercase letters indicate significant differences among different families (P < 0.05).
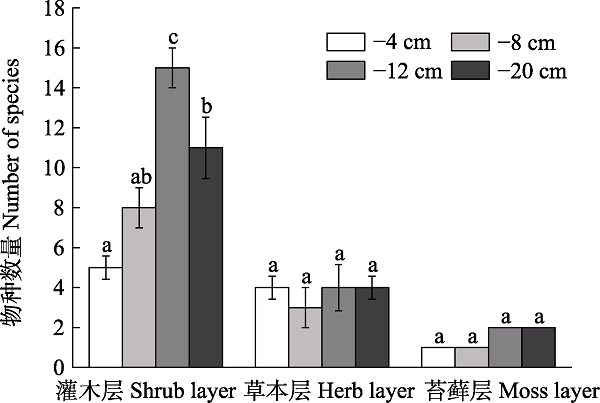
图3 鄂西南不同地下水位梯度下贫营养泥炭地灌木、草本和苔藓层物种数量(平均值± 标准误)。不同小写字母表示相同植被层不同水位梯度间存在显著差异(P < 0.05)。
Fig. 3 Number of species in shrub, herb and moss layers under different water table level gradients in oligotrophic peatland in the Southwestern Hubei Province (mean ± SE). Different lowercase letters indicate significant differences among different water table level gradients in the same vegetation layer (P < 0.05).
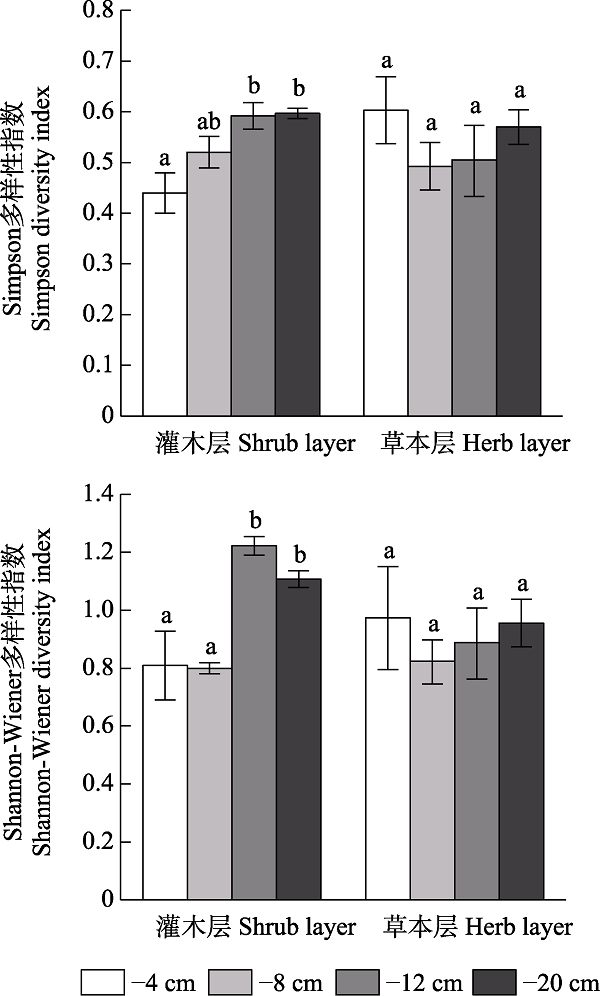
图4 鄂西南不同地下水位梯度下贫营养泥炭地灌木层和草本层物种多样性(平均值 ± 标准误)。不同小写字母表示相同植被层不同水位梯度间存在显著差异(P < 0.05)。
Fig. 4 Species diversity of shrub and herb layers under different water table level gradients in oligotrophic peatland in the Southwestern Hubei Province (mean ± SE). Different lowercase letters indicate significant differences among different water table level gradients in the same vegetation layer (P < 0.05).
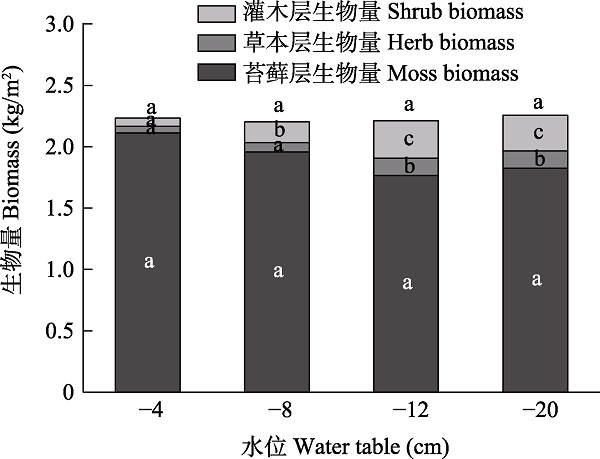
图5 鄂西南不同地下水位梯度下贫营养泥炭地灌木、草本和苔藓层生物量。不同小写字母表示相同植被层不同水位梯度间存在显著差异(P < 0.05)。
Fig. 5 Biomass of shrub, herb and moss layers under different water table level gradients in oligotrophic peatland in the Southwestern Hubei Province. Different lowercase letters indicate significant differences among different water table level gradients in the same vegetation layer (P < 0.05).
| [1] |
Aerts R, Verhoeven JTA, Whigham DF (1999) Plant-mediated controls on nutrient cycling in temperate fens and bogs. Ecology, 80, 2170-2181.
DOI URL |
| [2] |
Antala M, Juszczak R, Tol CVD, Rastogi A (2022) Impact of climate change-induced alterations in peatland vegetation phenology and composition on carbon balance. Science of the Total Environment, 827, 154294.
DOI URL |
| [3] |
Armstrong W, Justin SHFW, Beckett PM, Lythe S (1991) Root adaptation to soil waterlogging. Aquatic Botany, 39, 57-73.
DOI URL |
| [4] |
Bakker C, Bodegom VPM, Nelissen HJM, Aerts R, Ernst WHO (2007) Preference of wet dune species for waterlogged conditions can be explained by adaptations and specific recruitment requirements. Aquatic Botany, 86, 37-45.
DOI URL |
| [5] |
Bragazza L, Buttler A, Habermacher J, Brancaleoni L, Gerdol R, Fritze H, Hanajík P, Laiho R, Johnson D (2012) High nitrogen deposition alters the decomposition of bog plant litter and reduces carbon accumulation. Global Change Biology, 18, 1163-1172.
DOI URL |
| [6] |
Breeuwer A, Robroek BJM, Limpens J, Heijmans MMPD, Schouten MGC, Berendse F (2009) Decreased summer water table depth affects peatland vegetation. Basic and Applied Ecology, 10, 330-339.
DOI URL |
| [7] |
Buttler A, Robroek BJM, Laggoun DF, Jassey VEJ, Pochelon C, Bernard G, Delarue F, Gogo S, Mariotte P, Mitchell EAD (2015) Experimental warming interacts with soil moisture to discriminate plant responses in an ombrotrophic peatland. Journal of Vegetation Science, 26, 964-974.
DOI URL |
| [8] |
Chimner RA, Pypker TG, Hribljan JA, Moore PA, Waddington JM (2016) Multi-decadal changes in water table levels alter peatland carbon cycling. Ecosystems, 20, 1042-1057.
DOI URL |
| [9] | Clymo RS, Hayward PM (1982) The ecology of Sphagnum. In: Bryophyte Ecology (ed. Smith AJ), pp. 229-289. Springer, London. |
| [10] |
Dargie GC, Lewis SL, Lawson IT, Mitchard ET, Page SE, Bocko YE, Ifo SA (2017) Age, extent and carbon storage of the central Congo Basin peatland complex. Nature, 542, 86-90.
DOI |
| [11] |
Dieleman CM, Branfireun BA, McLaughlin JW, Lindo Z (2015) Climate change drives a shift in peatland ecosystem plant community: Implications for ecosystem function and stability. Global Change Biology, 21, 388-395.
DOI PMID |
| [12] | Dimitrov DD, Grant RF, Lafleur PM, Humphreys ER (2010) Modeling the effects of hydrology on gross primary productivity and net ecosystem productivity at Mer Bleue bog. Journal of Geophysical Research, 116, G04010. |
| [13] |
Dise NB (2009) Peatland response to global change. Science, 326, 810-811.
DOI URL |
| [14] |
Ellis T, Hill PW, Fenner N, Williams GG, Godbold D, Freeman C (2009) The interactive effects of elevated carbon dioxide and water table draw-down on carbon cycling in a Welsh ombrotrophic bog. Ecological Engineering, 35, 978-986.
DOI URL |
| [15] |
Evans CD, Peacock M, Baird AJ, Artz RRE, Burden A, Callaghan N, Chapman PJ, Cooper HM, Coyle M, Craig E, Cumming A, Dixon S, Gauci V, Grayson RP, Helfter C, Heppell CM, Holden J, Jones DL, Kaduk J, Levy P, Matthews R, McNamara NP, Misselbrook T, Oakley S, Page SE, Rayment M, Ridley LM, Stanley KM, Williamson JL, Worrall F, Morrison R (2021) Overriding water table control on managed peatland greenhouse gas emissions. Nature, 15, 1-20.
DOI |
| [16] |
Fenner N, Ostle N, Freeman Chris, Sleep D, Reynolds B (2004) Peatland carbon efflux partitioning reveals that Sphagnum photosynthate contributes to the DOC pool. Plant and Soil, 259, 345-354.
DOI URL |
| [17] | Freeman C, Ostle N, Kang H (2001) An enzymic ‘latch’ on a global carbon store. Nature, 409, 149. |
| [18] |
Gorham E (1991) Northern peatlands: Role in the carbon cycle and probable responses to climatic warming. Ecological Applications, 1, 182-195.
DOI URL |
| [19] |
Gudasz C, Bastviken D, Steger K, Premke K, Sobek S, Tranvik LJ (2010) Temperature-controlled organic carbon mineralization in lake sediments. Nature, 466, 478-481.
DOI |
| [20] |
Gunnarsson U (2005) Global patterns of Sphagnum productivity. Journal of Bryology, 27, 269-279.
DOI URL |
| [21] |
Halsey LA, Vitt DH, Gignac LD (2000) Sphagnum-dominated peatlands in North America since the last glacial maximum: Their occurrence and extent. Bryologist, 103, 334-352.
DOI URL |
| [22] |
Hogg EH, Maimer N, Wallen B (1994) Microsite and regional variation in the potential decay rate of Sphagnum magellanicum in South Swedish raised bogs. Ecography, 17, 50-59.
DOI URL |
| [23] | Hribljan JA, Kane ES, Pypker TG, Chimner RA (2014) The effect of long-term water table manipulations on dissolved organic carbon dynamics in a poor fen peatland. Journal of Geophysical Research, 119, 577-595. |
| [24] |
Jassey VEJ, Reczuga MK, Zielińska M, Słowińska S, Robroek BJM, Mariotte P, Seppey CVW, Lara E, Barabach J, Słowiński M (2018) Tipping point in plant-fungal interactions under severe drought causes abrupt rise in peatland ecosystem respiration. Global Change Biology, 24, 972-986.
DOI PMID |
| [25] |
Jassey VEJ, Signarbieux C (2019) Effects of climate warming on Sphagnum photosynthesis in peatlands depend on peat moisture and species-specific anatomical traits. Global Change Biology, 25, 3859-3870.
DOI URL |
| [26] | Johnson LC, Damman AWH (1991) Species-controlled Sphagnum decay on a south Swedish raised bog. Oikos, 61, 234-242. |
| [27] |
Kluge B, Wessolek G, Facklam M, Lorenz M, Schwärzel K (2008) Long-term carbon loss and CO2-C release of drained peatland soils in Northeast Germany. European Journal of Soil Science, 59, 1076-1086.
DOI URL |
| [28] |
Kozlowski TT (1986) Soil aeration and growth of forest trees. Scandinavian Journal of Forest Research, 1, 113-123.
DOI URL |
| [29] |
Laiho R (2006) Decomposition in peatlands: Reconciling seemingly contrasting results on the impacts of lowered water levels. Soil Biology and Biochemistry, 38, 2011-2024.
DOI URL |
| [30] |
Li CJ, Grayson R, Holden J, Li PF (2018) Erosion in peatlands: Recent research progress and future directions. Earth-Science Reviews, 185, 870-886.
DOI URL |
| [31] |
Limpens J, Berendse F, Blodau C, Canadell JG, Freeman C, Holden J, Roulet N, Rydin H, Gabriela SS (2008) Peatlands and the carbon cycle: From local processes to global implications—A synthesis. Biogeosciences, 5, 1475-1491.
DOI URL |
| [32] |
Liu LF, Chen H, Jiang L, Hu J, Zhan W, He YX, Zhu D, Zhong QP, Yang G (2018) Water table drawdown reshapes soil physicochemical characteristics in Zoige peatlands. Catena, 170, 119-128.
DOI URL |
| [33] | Liu XF, Yu XJ, Hong L, Wu L (2018) Distribution characteristics of soil organic carbon of the Sphagnum wetland in Qizimei Mountains of Southwestern Hubei. Hubei Forestry Science and Technology, 47(6), 21-26. (in Chinese with English abstract) |
| [刘雪飞, 余夏君, 洪柳, 吴林 (2018) 鄂西南七姊妹山两种泥炭藓湿地土壤有机碳分布特征的对比研究. 湖北林业科技, 47(6), 21-26.] | |
| [34] | Ma GL, Zhang QL, Zheng JX (2012) A review of ecological studies on Sphagnum mire in subtropical China. Journal of Anhui Agricultural Sciences, 40, 7859-7860. (in Chinese with English abstract) |
| [马广礼, 张巧莲, 郑俊霞 (2012) 亚热带泥炭藓沼泽生态学研究概述. 安徽农业科学, 40, 7859-7860.] | |
| [35] |
Ma XY, Xu H, Cao ZY, Shu L, Zhu RL (2022) Will climate change cause the global peatland to expand or contract? Evidence from the habitat shift pattern of Sphagnum mosses. Global Change Biology, 28, 6419-6432.
DOI URL |
| [36] |
Mäkiranta P, Laiho R, Mehtätalo L, Straková P, Sormunen J, Minkkinen K, Penttilä T, Fritze H, Tuittila ES (2018) Responses of phenology and biomass production of boreal fens to climate warming under different water-table level regimes. Global Change Biology, 24, 944-956.
DOI PMID |
| [37] | Malhotra A, Brice DJ, Childs J, Graham JD, Hobbie EA, Vander SH, Feron SC, Hanson PJ, Iversen CM (2020) Peatland warming strongly increases fine-root growth. Proceedings of the National Academy of Sciences, USA, 117, 17627-17634. |
| [38] | McNeil P, Waddington JM (2003) Moisture controls on Sphagnum growth and CO2 exchange on a cutover bog. Journal of Applied Ecology, 40, 354-367. |
| [39] |
McPartland MY, Kane ES, Falkowski MJ, Kolka R, Turetsky MR, Palik B, Montgomery RA (2019) The response of boreal peatland community composition and NDVI to hydrologic change, warming, and elevated carbon dioxide. Global Change Biology, 25, 93-107.
DOI PMID |
| [40] | Moore TR, Bubier JL, Frolking SE, Lafleur PM, Roulet NT (2002) Plant biomass and production and CO2 exchange in an ombrotrophic bog. Journal of Ecology, 90, 25-36. |
| [41] |
Murphy MT, Moore TR (2010) Linking root production to aboveground plant characteristics and water table in a temperate bog. Plant and Soil, 336, 219-231.
DOI URL |
| [42] |
Nieveen JP, Campbell DI, Schipper LA, Blair IJ (2005) Carbon exchange of grazed pasture on a drained peat soil. Global Change Biology, 11, 607-618.
DOI URL |
| [43] |
Pezeshki SR (2001) Wetland plant responses to soil flooding. Environmental and Experimental Botany, 46, 299-312.
DOI URL |
| [44] |
Potvin LR, Kane ES, Chimner RA, Kolka RK, Lilleskov EA (2015) Effects of water table position and plant functional group on plant community, aboveground production, and peat properties in a peatland mesocosm experiment (PEATcosm). Plant and Soil, 387, 277-294.
DOI URL |
| [45] |
Rousk J, Smith AR, Jones DL (2013) Investigating the long-term legacy of drought and warming on the soil microbial community across five European shrubland ecosystems. Global Change Biology, 19, 3872-3884.
DOI PMID |
| [46] |
Rydin H, McDonald AJS (1985) Tolerance of Sphagnum to water level. Journal of Bryology, 13, 571-578.
DOI URL |
| [47] |
Shannon CE (1948) A mathematical theory of communication. The Bell System Technical Journal, 27, 379-423.
DOI URL |
| [48] |
Silvola J, Alm J, Ahlholm U, Nykanen H, Martikainen PJ (1996) CO2 fluxes from peat in boreal mires under varying temperature and moisture conditions. Journal of Ecology, 84, 219-228.
DOI URL |
| [49] |
Simola H, Pitkänen A, Turunen J (2012) Carbon loss in drained forestry peatlands in Finland, estimated by resampling peatlands surveyed in the 1980s. European Journal of Soil Science, 63, 798-807.
DOI URL |
| [50] |
Simpson EH (1949) Measurement of diversity. Nature, 163, 688-688.
DOI |
| [51] |
Sonesson M, Carlsson BÅ, Callaghan TV, Halling S, Björn LO, Bertgren M, Johanson U (2002) Growth of two peat-forming mosses in subarctic mires: Species interactions and effects of simulated climate change. Oikos, 99, 151-160.
DOI URL |
| [52] |
Strack M, Waddington JM, Bourbonniere RA, Buckton EL, Shaw K, Whittington P, Price JS (2008) Effect of water table drawdown on peatland dissolved organic carbon export and dynamics. Hydrological Processes, 22, 3373-3385.
DOI URL |
| [53] | Strack M, Waddington JM, Rochefort L, Tuittila ES (2006) Response of vegetation and net ecosystem carbon dioxide exchange at different peatland microforms following water table drawdown. Journal of Geophysical Research, 111, G02006. |
| [54] |
Strack M, Zuback Y, McCarter C, Price J (2015) Changes in dissolved organic carbon quality in soils and discharge 10 years after peatland restoration. Journal of Hydrology, 527, 345-354.
DOI URL |
| [55] |
Straková P, Anttila J, Spetz P, Kitunen V, Tapanila T, Laiho R (2010) Litter quality and its response to water level drawdown in boreal peatlands at plant species and community level. Plant and Soil, 335, 501-520.
DOI URL |
| [56] |
Sulman BN, Desai AR, Cook BD, Saliendra N, Mackay DS (2009) Contrasting carbon dioxide fluxes between a drying shrub wetland in Northern Wisconsin, USA, and nearby forests. Biogeosciences, 6, 1115-1126.
DOI URL |
| [57] |
Talbot J, Richard PJH, Roulet NT, Booth RK (2010) Assessing long-term hydrological and ecological responses to drainage in a raised bog using paleoecology and a hydrosequence. Journal of Vegetation Science, 21, 143-156.
DOI URL |
| [58] | Turetsky MR, Treat CC, Waldrop MP, Waddington JM, Harden JW, McGuire AD (2008) Short-term response of methane fluxes and methanogen activity to water table and soil warming manipulations in an Alaskan peatland. Journal of Geophysical Research, 113, G00A10. |
| [59] | Turetsky MR, Donahue WF, Benscoter BW (2011) Experimental drying intensifies burning and carbon losses in a northern peatland. Nature Communications, 2, 1523. |
| [60] |
Waddington JM, Rochefort L, Campeau S (2003) Sphagnum production and decomposition in a restored cutover peatland. Wetlands Ecology and Management, 11, 85-95.
DOI URL |
| [61] | Wang H, Wu L, Xue D, Liu XF, Hong L, Mou L, Li XL (2020) Distribution and environmental characteristics of sphagnum peat bogs in Taishanmiao in Enshi City, Hubei Province. Wetland Science, 18, 266-274. (in Chinese with English abstract) |
| [王涵, 吴林, 薛丹, 刘雪飞, 洪柳, 牟利, 李小玲 (2020) 湖北省恩施市太山庙泥炭藓泥炭沼泽分布及其环境特征研究. 湿地科学, 18, 266-274.] | |
| [62] |
Wang H, Yu LF, Zhang ZH, Liu W, Chen LT, Cao GG, Yue HW, Zhou JZ, Yang YF, Tang YH (2017) Molecular mechanisms of water table lowering and nitrogen deposition in affecting greenhouse gas emissions from a Tibetan alpine wetland. Global Change Biology, 23, 815-829.
DOI PMID |
| [63] |
Ward SE, Bardgett RD, McNamara NP, Ostle NJ (2009) Plant functional group identity influences short-term peatland ecosystem carbon flux: Evidence from a plant removal experiment. Functional Ecology, 23, 454-462.
DOI URL |
| [64] |
Weltzin JF, Bridgham SD, Pastor J, Chen J, Harth C (2003) Potential effects of warming and drying on peatland plant community composition. Global Change Biology, 9, 141-151.
DOI URL |
| [65] |
Weltzin JF, Harth C Bridgham SD, Pastor J, Vonderharr M (2001) Production and microtopography of bog bryophytes: Response to warming and water-table manipulations. Oecologia, 128, 557-565.
DOI PMID |
| [66] |
Weltzin JF, Pastor J, Harth C, Bridgham SD, Updegraff K, Chapin CT (2000) Response of bog and fen plant communities to warming and water-table manipulations. Ecology, 81, 3464-3478.
DOI URL |
| [67] |
Wang YY, Wang H, He JS, Feng XJ (2017) Iron-mediated soil carbon response to water-table decline in an alpine wetland. Nature Communications, 8, 15972.
DOI PMID |
| [68] |
Wu GL, Ren GH, Wang D, Shi ZH, Warrington D (2013) Above and below-ground response to soil water change in an alpine wetland ecosystem on the Qinghai-Tibetan Plateau, China. Journal of Hydrology, 476, 120-127.
DOI URL |
| [69] | Yang G, Wen XR, Bai YP, Lu J, Liu YZ (2018) Review of accumulation differences of phenolic compounds and their effect on carbon export in degradation peatland. Ecological Science, 37, 229-232. (in Chinese with English abstract) |
| [杨刚, 温晓荣, 白银萍, 芦静, 刘银占 (2018) 酚类物质对退化泥炭地碳输出的影响研究进展. 生态科学, 37, 229-232.] | |
| [70] | Yu ZC, Loisel J, Brosseau DP, Beilman DW, Hunt SJ (2010) Global peatland dynamics since the last glacial maximum. Geophysical Research Letters, 37, L13402. |
| [71] | Zeng J, Chen H, Liu JL, Yang SZ, Yan F, Cao Q, Yang G (2022) The decrease of peatland water table on the Qinghai-Tibet Plateau caused the increase of soil phenolic substances and vegetation biomass which promoted the accumulation of soil carbon. Acta Ecologica Sinica, 42, 625-634. (in Chinese with English abstract) |
| [曾嘉, 陈槐, 刘建亮, 杨随庄, 严飞, 曹芹, 杨刚 (2022) 青藏高原泥炭地水位下降促进土壤碳积累的影响机制. 生态学报, 42, 625-634.] | |
| [72] |
Zhang QG, Zhang DY (2003) Biodiversity and ecosystem functioning: Recent advances and trends. Biodiversity Science, 11, 351-363. (in Chinese with English abstract)
DOI URL |
|
[张全国, 张大勇 (2003) 生物多样性与生态系统功能: 最新的进展与动向. 生物多样性, 11, 351-363.]
DOI |
|
| [73] | Zhou WC, SuoLang DEJ, Cui LJ, Wang YF, Li W (2016) Effects of drainage on soil organic carbon stock in the Zoige peatlands, eastern Qinghai-Tibetan Plateau. Acta Ecologica Sinica, 36, 2123-2132. (in Chinese with English abstract) |
| [周文昌, 索郎夺尔基, 崔丽娟, 王义飞, 李伟 (2016) 排水对若尔盖高原泥炭地土壤有机碳储量的影响. 生态学报, 36, 2123-2132.] | |
| [74] | Zhu RL (2022) Peat mosses (Sphagnum): Ecologically, economically, and scientifically important group of carbon sequestration plants. Chinese Bulletin of Botany, 57, 559-578. (in Chinese with English abstract) |
|
[朱瑞良 (2022) 泥炭藓: 一类具有重要生态、经济和科学价值的碳封存植物. 植物学报, 57, 559-578.]
DOI |
| [1] | 吴晓晴 张美惠 葛苏婷 李漫淑 宋坤 沈国春 达良俊 张健. 上海近自然林重建过程中木本植物物种多样性与地上生物量的时空动态——以闵行区生态岛为例[J]. 生物多样性, 2025, 33(5): 24444-. |
| [2] | 张浩斌, 肖路, 刘艳杰. 夜间灯光对外来入侵植物和本地植物群落多样性和生长的影响[J]. 生物多样性, 2025, 33(4): 24553-. |
| [3] | 宋威, 程才, 王嘉伟, 吴纪华. 土壤微生物对植物多样性–生态系统功能关系的调控作用[J]. 生物多样性, 2025, 33(4): 24579-. |
| [4] | 吴昱萱, 王平, 胡晓生, 丁一, 彭甜恬, 植秋滢, 巴德木其其格, 李文杰, 关潇, 李俊生. 呼伦贝尔草地退化现状评估与植被特征变化[J]. 生物多样性, 2025, 33(2): 24118-. |
| [5] | 尹星元, 安慧, 邢彬彬, 苏诗玉, 文志林, 郭建超, 刘小平, 王波. 养分添加和降水变化对荒漠草原地上和地下生物量稳定性的影响[J]. 生物多样性, 2024, 32(7): 24073-. |
| [6] | 连佳丽, 陈婧, 杨雪琴, 赵莹, 罗叙, 韩翠, 赵雅欣, 李建平. 荒漠草原植物多样性和微生物多样性对降水变化的响应[J]. 生物多样性, 2024, 32(6): 24044-. |
| [7] | 吴乐婕, 刘泽康, 田星, 张群, 李博, 吴纪华. 海三棱藨草基因型多样性对种群营养生长和繁殖策略的影响[J]. 生物多样性, 2024, 32(4): 23478-. |
| [8] | 万凤鸣, 万华伟, 张志如, 高吉喜, 孙晨曦, 王永财. 草地植物多样性无人机调查的应用潜力[J]. 生物多样性, 2024, 32(3): 23381-. |
| [9] | 张乃鹏, 梁洪儒, 张焱, 孙超, 陈勇, 王路路, 夏江宝, 高芳磊. 土壤类型和地下水埋深对黄河三角洲典型盐沼植物群落空间分异的影响[J]. 生物多样性, 2024, 32(2): 23370-. |
| [10] | 王兴煜, 孟京辉, 任思远, 祝燕. 北京东灵山暖温带落叶阔叶林群落生物多样性与地上生物量的关系[J]. 生物多样性, 2024, 32(12): 24230-. |
| [11] | 蒋陈焜, 郁文彬, 饶广远, 黎怀成, Julien B. Bachelier, Hartmut H. Hilger, Theodor C. H. Cole. 植物系统发生海报——以演化视角介绍植物多样性的科教资料项目[J]. 生物多样性, 2024, 32(11): 24210-. |
| [12] | 韩赟, 迟晓峰, 余静雅, 丁旭洁, 陈世龙, 张发起. 青海野生维管植物名录[J]. 生物多样性, 2023, 31(9): 23280-. |
| [13] | 陈又生, 宋柱秋, 卫然, 罗艳, 陈文俐, 杨福生, 高连明, 徐源, 张卓欣, 付鹏程, 向春雷, 王焕冲, 郝加琛, 孟世勇, 吴磊, 李波, 于胜祥, 张树仁, 何理, 郭信强, 王文广, 童毅华, 高乞, 费文群, 曾佑派, 白琳, 金梓超, 钟星杰, 张步云, 杜思怡. 西藏维管植物多样性编目和分布数据集[J]. 生物多样性, 2023, 31(9): 23188-. |
| [14] | 宋柱秋, 叶文, 董仕勇, 金梓超, 钟星杰, 王震, 张步云, 徐晔春, 陈文俐, 李世晋, 姚纲, 徐洲锋, 廖帅, 童毅华, 曾佑派, 曾云保, 陈又生. 广东省高等植物多样性编目和分布数据集[J]. 生物多样性, 2023, 31(9): 23177-. |
| [15] | 梁彩群, 陈玉凯, 杨小波, 张凯, 李东海, 江悦馨, 李婧涵, 王重阳, 张顺卫, 朱子丞. 海南省野生维管植物编目和分布数据集[J]. 生物多样性, 2023, 31(6): 23067-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn