



生物多样性 ›› 2019, Vol. 27 ›› Issue (8): 842-853. DOI: 10.17520/biods.2019034 cstr: 32101.14.biods.2019034
收稿日期:2019-02-14
接受日期:2019-05-15
出版日期:2019-08-20
发布日期:2019-05-20
通讯作者:
王龙
基金资助:
Zhongdong Yu1,Zhihe Yu2,Shiyu Jin3,Long Wang4,*( )
)
Received:2019-02-14
Accepted:2019-05-15
Online:2019-08-20
Published:2019-05-20
Contact:
Wang Long
摘要:
黄曲霉(Aspergillus flavus)是一种广泛分布的腐生真菌, 是黄曲霉毒素B (aflatoxin B, AFB)和圆弧偶氮酸(cyclopiazonic acid, CPA)的主要产生菌, 也是动植物的条件致病菌。全球的玉米、花生和棉籽均不同程度地遭到黄曲霉及其毒素的污染。黄曲霉菌株间在形态学、遗传学和产毒特性上变异较大, 且其居群遗传结构也尚不明确。为了揭示黄曲霉居群遗传结构及其产毒素特性的规律, 本研究选取了从我国26省区(包括大小兴安岭)不同环境中分离的黄曲霉88株, 结合模式菌株和国际权威菌株9株, 基于钙调蛋白基因(CaM)和β-微管蛋白基因(benA)进行多基因序列分型(multi-locus sequence typing, MLST), 使用MEGA 6.0和Structure 2.3.4软件进行系统发育学分析和居群结构推导, 并结合菌株的产毒特性(AFB和CPA)进行比较分析。结果显示本研究的97株黄曲霉可分为3个居群, 即黄曲霉居群I、黄曲霉居群II和米曲霉居群, 该97株黄曲霉共有17个序列型(sequence type, ST), 其中我国的88株菌分布于15个序列型。米曲霉居群均不产AFB, 黄曲霉居群I和II的菌株绝大多数都产AFB和CPA, 其产毒特性只具有菌株特异性, 与居群和序列型无关。黄曲霉菌株产毒特性与地理分布或农作物类型间存在一定关系。我国东北玉米产区、西北干旱棉花产区和南方花生产区的黄曲霉居群I和II菌株均产AFB和CPA, 我国青海可可西里和四川阿坝地区的黄曲霉仅产CPA而不产AFB, 不产AFB的米曲霉居群大部分来自我国气候和地理环境多样的华北地区, 该地区也是我国农村传统酿造黄豆酱的地区。
余仲东, 余知和, 金世宇, 王龙 (2019) 我国黄曲霉遗传多样性与产毒特性. 生物多样性, 27, 842-853. DOI: 10.17520/biods.2019034.
Zhongdong Yu, Zhihe Yu, Shiyu Jin, Long Wang (2019) Genetic diversity and toxin-producing characters of Aspergillus flavus from China. Biodiversity Science, 27, 842-853. DOI: 10.17520/biods.2019034.
| 菌株顺序号* Number | 物种 Species | 菌株 Strains | 分离地和基物 Isolation places and substrates | 产毒素 Toxin production | |
|---|---|---|---|---|---|
| AFB | CPA | ||||
| 1 | A. flavus | CBS 100927T | 南太平洋群岛; 赛璐玢 Cellophane; South Pacific Islands | - | + |
| 2 | A. oryzae | CBS 100925T | 日本大阪; 分离基物未知 Ex-type of A. oryzae, unknown source; Osaka, Japan | - | + |
| 3 | A. thomii | CBS 120.51T | 英国伦敦; 污染物 Ex-type of A. thomii, culture contaminant; London, UK | - | + |
| 4 | A. flavus | CYH2-2-1 | 河北石家庄; 空气 Air; Shijiazhuang, Hebei, China | + | + |
| 5 | A. flavus | 3.4408 | 日本东京; 土壤 Soil; Tokyo, Japan | + | + |
| 6 | A. flavus | 14527 | 西藏米林; 土壤 Soil; Milin, Tibet, China | + | + |
| 7 | A. flavus | 13483 | 山西五台山; 土壤 Soil; Mt. Wutaishan, Shanxi, China | + | + |
| 8 | A. flavus | 13868 | 内蒙古呼伦贝尔; 土壤 Soil; Hulun Buir, Inner Mongolia, China | + | + |
| 9 | A. flavus | 13894 | 湖南益阳; 土壤 Soil; Yiyang, Hunan, China | + | + |
| 10 | A. flavus | 13895 | 江西三清山; 土壤 Soil; Sanqingshan, Jiangxi, China | + | + |
| 11 | A. flavus | 13918 | 江苏苏州; 土壤 Soil, Suzhou, Jiangsu, China | + | + |
| 12 | A. flavus | 13952 | 甘肃兰州; 土壤 Soil; Lanzhou, Gansu, China | - | + |
| 13 | A. flavus | 13961 | 宁夏罗山; 土壤 Soil; Luoshan, Ningxia, China | - | - |
| 14 | A. flavus | 13962 | 宁夏灵武; 土壤 Soil; Lingwu, Ningxia, China | - | - |
| 15 | A. flavus | 14099 | 山西吕梁; 土壤 Soil; lvliang, Shanxi, China | + | + |
| 16 | A. flavus | 14131 | 山西大同; 土壤 Soil; Datong, Shanxi, China | + | + |
| 17 | A. flavus | 14151 | 河南洛阳; 土壤 Soil; Luoyang, Henan, China | - | - |
| 18 | A. flavus | 14152 | 河南南阳; 土壤 Soil; Nanyang, Henan, China | - | - |
| 19 | A. flavus | 14153 | 山东泰安 土壤 Soil; Tai’an, Shandong, China | - | - |
| 20 | A. flavus | 14154 | 山东临沂; 土壤 Soil; Linyi, Shandong, China | - | - |
| 21 | A. flavus | 14155 | 河北兴隆; 土壤 Soil; Xinglong, Hebei, China | - | - |
| 22 | A. flavus | 14156 | 河北张家口; 土壤 Soil; Zhangjiakou, Hebei, China | - | - |
| 23 | A. flavus | 14157 | 河北保定; 土壤 Soil; Baoding, Hebei, China | - | - |
| 24 | A. flavus | 14159 | 河北衡水; 土壤 Soil; Hengshui, Hebei, China | - | - |
| 25 | A. flavus | 14175 | 浙江乌镇; 土壤 Soil; Wuzhen, Zhejiang, China | + | + |
| 26 | A. flavus | 14334 | 安徽巢湖; 荸荠 Water chestnut; Chaohu, Anhui, China | + | + |
| 27 | A. flavus | 14353 | 新疆吐鲁番; 土壤 Soil; Turpan, Xinjiang, China | + | + |
| 28 | A. flavus | 14355 | 新疆石河子; 土壤 Soil; Shihezi, Xinjiang, China | + | + |
| 29 | A. flavus | 14356 | 新疆乌鲁木齐; 土壤 Soil; Urumqi, Xinjiang, China | + | + |
| 30 | A. flavus | 14357 | 新疆伊犁; 土壤 Soil; Yili, Xinjiang, China | + | + |
| 31 | A. flavus | 14358 | 陕西榆林; 土壤 Soil; Yulin, Shaanxi, China | + | + |
| 32 | A. flavus | 14359 | 陕西汉中; 土壤 Soil; Hanzhong, Shaanxi, China | + | + |
| 33 | A. flavus | 14373 | 陕西渭南; 土壤 Soil; Weinan, Shaanxi, China | + | + |
| 34 | A. flavus | 14374 | 新疆吐鲁番; 土壤 Soil, Turpan, Xinjiang, China | + | + |
| 35 | A. flavus | 23124 | 海南五指山; 土壤 Soil; Mt. Wuzhishan, Hainan, China | + | + |
表1 黄曲霉菌株、分离地和基物及产毒特性
Table 1 Aspergillus flavus strains, isolation places and toxin-production
| 菌株顺序号* Number | 物种 Species | 菌株 Strains | 分离地和基物 Isolation places and substrates | 产毒素 Toxin production | |
|---|---|---|---|---|---|
| AFB | CPA | ||||
| 1 | A. flavus | CBS 100927T | 南太平洋群岛; 赛璐玢 Cellophane; South Pacific Islands | - | + |
| 2 | A. oryzae | CBS 100925T | 日本大阪; 分离基物未知 Ex-type of A. oryzae, unknown source; Osaka, Japan | - | + |
| 3 | A. thomii | CBS 120.51T | 英国伦敦; 污染物 Ex-type of A. thomii, culture contaminant; London, UK | - | + |
| 4 | A. flavus | CYH2-2-1 | 河北石家庄; 空气 Air; Shijiazhuang, Hebei, China | + | + |
| 5 | A. flavus | 3.4408 | 日本东京; 土壤 Soil; Tokyo, Japan | + | + |
| 6 | A. flavus | 14527 | 西藏米林; 土壤 Soil; Milin, Tibet, China | + | + |
| 7 | A. flavus | 13483 | 山西五台山; 土壤 Soil; Mt. Wutaishan, Shanxi, China | + | + |
| 8 | A. flavus | 13868 | 内蒙古呼伦贝尔; 土壤 Soil; Hulun Buir, Inner Mongolia, China | + | + |
| 9 | A. flavus | 13894 | 湖南益阳; 土壤 Soil; Yiyang, Hunan, China | + | + |
| 10 | A. flavus | 13895 | 江西三清山; 土壤 Soil; Sanqingshan, Jiangxi, China | + | + |
| 11 | A. flavus | 13918 | 江苏苏州; 土壤 Soil, Suzhou, Jiangsu, China | + | + |
| 12 | A. flavus | 13952 | 甘肃兰州; 土壤 Soil; Lanzhou, Gansu, China | - | + |
| 13 | A. flavus | 13961 | 宁夏罗山; 土壤 Soil; Luoshan, Ningxia, China | - | - |
| 14 | A. flavus | 13962 | 宁夏灵武; 土壤 Soil; Lingwu, Ningxia, China | - | - |
| 15 | A. flavus | 14099 | 山西吕梁; 土壤 Soil; lvliang, Shanxi, China | + | + |
| 16 | A. flavus | 14131 | 山西大同; 土壤 Soil; Datong, Shanxi, China | + | + |
| 17 | A. flavus | 14151 | 河南洛阳; 土壤 Soil; Luoyang, Henan, China | - | - |
| 18 | A. flavus | 14152 | 河南南阳; 土壤 Soil; Nanyang, Henan, China | - | - |
| 19 | A. flavus | 14153 | 山东泰安 土壤 Soil; Tai’an, Shandong, China | - | - |
| 20 | A. flavus | 14154 | 山东临沂; 土壤 Soil; Linyi, Shandong, China | - | - |
| 21 | A. flavus | 14155 | 河北兴隆; 土壤 Soil; Xinglong, Hebei, China | - | - |
| 22 | A. flavus | 14156 | 河北张家口; 土壤 Soil; Zhangjiakou, Hebei, China | - | - |
| 23 | A. flavus | 14157 | 河北保定; 土壤 Soil; Baoding, Hebei, China | - | - |
| 24 | A. flavus | 14159 | 河北衡水; 土壤 Soil; Hengshui, Hebei, China | - | - |
| 25 | A. flavus | 14175 | 浙江乌镇; 土壤 Soil; Wuzhen, Zhejiang, China | + | + |
| 26 | A. flavus | 14334 | 安徽巢湖; 荸荠 Water chestnut; Chaohu, Anhui, China | + | + |
| 27 | A. flavus | 14353 | 新疆吐鲁番; 土壤 Soil; Turpan, Xinjiang, China | + | + |
| 28 | A. flavus | 14355 | 新疆石河子; 土壤 Soil; Shihezi, Xinjiang, China | + | + |
| 29 | A. flavus | 14356 | 新疆乌鲁木齐; 土壤 Soil; Urumqi, Xinjiang, China | + | + |
| 30 | A. flavus | 14357 | 新疆伊犁; 土壤 Soil; Yili, Xinjiang, China | + | + |
| 31 | A. flavus | 14358 | 陕西榆林; 土壤 Soil; Yulin, Shaanxi, China | + | + |
| 32 | A. flavus | 14359 | 陕西汉中; 土壤 Soil; Hanzhong, Shaanxi, China | + | + |
| 33 | A. flavus | 14373 | 陕西渭南; 土壤 Soil; Weinan, Shaanxi, China | + | + |
| 34 | A. flavus | 14374 | 新疆吐鲁番; 土壤 Soil, Turpan, Xinjiang, China | + | + |
| 35 | A. flavus | 23124 | 海南五指山; 土壤 Soil; Mt. Wuzhishan, Hainan, China | + | + |
| 菌株顺序号* Number | 物种 Species | 菌株 Strains | 分离地和基物 Isolation places and substrates | 产毒素 Toxin production | |
|---|---|---|---|---|---|
| AFB | CPA | ||||
| 36 | A. flavus | AB34 | 四川若尔盖; 土壤 Soil; Ruoergai Prairie, Sichuan, China | - | + |
| 37 | A. flavus | FJ17-2 | 福建宁德; 茶叶 Tea; Ningde, Fujian, China | + | + |
| 38 | A. flavus | HB4 | 湖北神农架; 土壤 Soil; Shennongjia, Hubei, China | + | + |
| 39 | A. flavus | HL53 | 黑龙江凉水; 土壤 Soil; Liangshui Nature Reserve, Heilongjiang, China | + | + |
| 40 | A. flavus | HL70 | 黑龙江乌伊岭; 土壤 Soil; Wuyiling, Heilongjiang, China | + | + |
| 41 | A. flavus | KK39 | 青海互助县; 土壤 Soil; Huzhu County, Qinghai, China | - | + |
| 42 | A. flavus | KK41 | 青海互助县; 土壤 Soil; Huzhu County, Qinghai, China | - | + |
| 43 | A. flavus | KK49 | 青海互助县; 土壤 Soil; Huzhu County, Qinghai, China | - | + |
| 44 | A. flavus | KK50 | 青海可可西里; 土壤 Soil; Hoh Xil, Qinghai, China | - | + |
| 45 | A. flavus | KK65 | 青海楚玛尔河; 土壤 Soil; Chumaer River, Qinghai, China | - | + |
| 46 | A. flavus | KK66 | 青海可可西里; 土壤 Soil; Hoh Xil, Qinghai, China | - | + |
| 47 | A. flavus | KK67 | 青海楚玛尔河; 土壤 Soil; Chumaer River, Qinghai, China | - | + |
| 48 | A. flavus | KK68 | 青海沱沱河; 土壤 Soil; Tuotuo River, Qinghai, China | - | + |
| 49 | A. flavus | KK69 | 青海沱沱河; 土壤 Soil; Tuotuo River, Qinghai, China | - | + |
| 50 | A. flavus | KK70 | 青海楚玛尔河; 土壤 Soil; Chumaer River, Qinghai, China | - | + |
| 51 | A. flavus | KK72 | 青海青海湖; 土壤 Soil; Qinghai Lake, Qinghai, China | + | + |
| 52 | A. flavus | KK73 | 青海青海湖; 土壤 Soil; Qinghai Lake, Qinghai, China | + | + |
| 53 | A. flavus | KK94 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | + | + |
| 54 | A. flavus | KK102 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 55 | A. flavus | KK103 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 56 | A. flavus | KK104 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 57 | A. flavus | KK114 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 58 | A. flavus | XZ107 | 陕西南宫山; 植物叶 Plant leaves; Mt. Nangongshan, Shaanxi, China | + | + |
| 59 | A. flavus | XZ108 | 陕西通天河; 植物叶 Plant leaves; Tongtian River, Shaanxi, China | + | + |
| 60 | A. flavus | XZ109 | 陕西南宫山; 植物叶 Plant leaves; Mt. Nangongshan, Shaanxi, China | + | + |
| 61 | A. flavus | XZ112 | 陕西通天河; 植物叶 Plant leaves; Tongtian River, Shaanxi, China | + | + |
| 62 | A. flavus | YN23 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | - | + |
| 63 | A. flavus | YN35 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 64 | A. flavus | YN48 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 65 | A. flavus | YN49 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 66 | A. flavus | YN51 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 67 | A. flavus | NRRL 3357 | 美国; 霉花生 Moldy peanuts; USA | + | + |
| 68 | A. oryzae | RIB40 | 日本; 谷粒 Cereal grains; Japan | - | - |
| 69 | A. flavus | 3.262 | 辽宁大连; 空气 Air; Dalian, Liaoning, China | - | - |
| 70 | A. flavus | 3.267 | 辽宁大连; 土壤 Soil; Dalian, Liaoning, China | - | + |
| 71 | A. flavus | 3.337 | 天津; 蚊香 Mosquito-repellent incense; Tianjin, China | - | + |
| 72 | A. flavus | 3.417 | 天津; 酱曲 Soy sauce starter; Tianjin, China | - | + |
| 73 | A. flavus | 3.870 | 天津; 酱曲 Soy sauce starter; Tianjin, China | + | + |
| 74 | A. flavus | 3.881 | 上海; 小麦 Wheat; Shanghai, China | - | + |
| 75 | A. flavus | 3.2146 | 北京; 大米 Rice; Beijing, China | + | + |
| 76 | A. flavus | 3.2758 | 广东广州; 空气 Air; Guangzhou, Guangdong, China | - | + |
| 77 | A. flavus | 3.2789 | 越南河内; 土壤 Soil; Hanoi, Vietnam | - | + |
| 78 | A. flavus | 3.2823 | 安徽芜湖; 植物 Plants; Wuhu, Anhui, China | - | + |
表1 (续)
Table 1 (continued)
| 菌株顺序号* Number | 物种 Species | 菌株 Strains | 分离地和基物 Isolation places and substrates | 产毒素 Toxin production | |
|---|---|---|---|---|---|
| AFB | CPA | ||||
| 36 | A. flavus | AB34 | 四川若尔盖; 土壤 Soil; Ruoergai Prairie, Sichuan, China | - | + |
| 37 | A. flavus | FJ17-2 | 福建宁德; 茶叶 Tea; Ningde, Fujian, China | + | + |
| 38 | A. flavus | HB4 | 湖北神农架; 土壤 Soil; Shennongjia, Hubei, China | + | + |
| 39 | A. flavus | HL53 | 黑龙江凉水; 土壤 Soil; Liangshui Nature Reserve, Heilongjiang, China | + | + |
| 40 | A. flavus | HL70 | 黑龙江乌伊岭; 土壤 Soil; Wuyiling, Heilongjiang, China | + | + |
| 41 | A. flavus | KK39 | 青海互助县; 土壤 Soil; Huzhu County, Qinghai, China | - | + |
| 42 | A. flavus | KK41 | 青海互助县; 土壤 Soil; Huzhu County, Qinghai, China | - | + |
| 43 | A. flavus | KK49 | 青海互助县; 土壤 Soil; Huzhu County, Qinghai, China | - | + |
| 44 | A. flavus | KK50 | 青海可可西里; 土壤 Soil; Hoh Xil, Qinghai, China | - | + |
| 45 | A. flavus | KK65 | 青海楚玛尔河; 土壤 Soil; Chumaer River, Qinghai, China | - | + |
| 46 | A. flavus | KK66 | 青海可可西里; 土壤 Soil; Hoh Xil, Qinghai, China | - | + |
| 47 | A. flavus | KK67 | 青海楚玛尔河; 土壤 Soil; Chumaer River, Qinghai, China | - | + |
| 48 | A. flavus | KK68 | 青海沱沱河; 土壤 Soil; Tuotuo River, Qinghai, China | - | + |
| 49 | A. flavus | KK69 | 青海沱沱河; 土壤 Soil; Tuotuo River, Qinghai, China | - | + |
| 50 | A. flavus | KK70 | 青海楚玛尔河; 土壤 Soil; Chumaer River, Qinghai, China | - | + |
| 51 | A. flavus | KK72 | 青海青海湖; 土壤 Soil; Qinghai Lake, Qinghai, China | + | + |
| 52 | A. flavus | KK73 | 青海青海湖; 土壤 Soil; Qinghai Lake, Qinghai, China | + | + |
| 53 | A. flavus | KK94 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | + | + |
| 54 | A. flavus | KK102 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 55 | A. flavus | KK103 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 56 | A. flavus | KK104 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 57 | A. flavus | KK114 | 青海坎布拉; 土壤 Soil; Kanbula, Qinghai, China | - | + |
| 58 | A. flavus | XZ107 | 陕西南宫山; 植物叶 Plant leaves; Mt. Nangongshan, Shaanxi, China | + | + |
| 59 | A. flavus | XZ108 | 陕西通天河; 植物叶 Plant leaves; Tongtian River, Shaanxi, China | + | + |
| 60 | A. flavus | XZ109 | 陕西南宫山; 植物叶 Plant leaves; Mt. Nangongshan, Shaanxi, China | + | + |
| 61 | A. flavus | XZ112 | 陕西通天河; 植物叶 Plant leaves; Tongtian River, Shaanxi, China | + | + |
| 62 | A. flavus | YN23 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | - | + |
| 63 | A. flavus | YN35 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 64 | A. flavus | YN48 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 65 | A. flavus | YN49 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 66 | A. flavus | YN51 | 云南玉溪; 烟叶 Tobacco leaves; Yuxi, Yunnan, China | + | + |
| 67 | A. flavus | NRRL 3357 | 美国; 霉花生 Moldy peanuts; USA | + | + |
| 68 | A. oryzae | RIB40 | 日本; 谷粒 Cereal grains; Japan | - | - |
| 69 | A. flavus | 3.262 | 辽宁大连; 空气 Air; Dalian, Liaoning, China | - | - |
| 70 | A. flavus | 3.267 | 辽宁大连; 土壤 Soil; Dalian, Liaoning, China | - | + |
| 71 | A. flavus | 3.337 | 天津; 蚊香 Mosquito-repellent incense; Tianjin, China | - | + |
| 72 | A. flavus | 3.417 | 天津; 酱曲 Soy sauce starter; Tianjin, China | - | + |
| 73 | A. flavus | 3.870 | 天津; 酱曲 Soy sauce starter; Tianjin, China | + | + |
| 74 | A. flavus | 3.881 | 上海; 小麦 Wheat; Shanghai, China | - | + |
| 75 | A. flavus | 3.2146 | 北京; 大米 Rice; Beijing, China | + | + |
| 76 | A. flavus | 3.2758 | 广东广州; 空气 Air; Guangzhou, Guangdong, China | - | + |
| 77 | A. flavus | 3.2789 | 越南河内; 土壤 Soil; Hanoi, Vietnam | - | + |
| 78 | A. flavus | 3.2823 | 安徽芜湖; 植物 Plants; Wuhu, Anhui, China | - | + |
| 菌株顺序号* Number | 物种 Species | 菌株 Strains | 分离地和基物 Isolation places and substrates | 产毒素 Toxin production | |
|---|---|---|---|---|---|
| AFB | CPA | ||||
| 79 | A. flavus | 3.3554 | 北京; 空气 Air; Beijing, China | - | + |
| 80 | A. flavus var. columnaris | CBS 485.65T | 日本; 黄油 Ex-type of A. flavus var. columnaris, butter; Japan | - | + |
| 81 | A. flavus | 3.4408-2 | 北京; 空气 Air; Beijing, China | + | + |
| 82 | A. flavus | 3.4410 | 美国 ATCC 28539; USA | + | + |
| 83 | A. flavus | 3.5211 | 北京 CICC 2348; Beijing, China | - | + |
| 84 | A. flavus | 3.5278 | 四川德阳; 烂水果 Rotten fruit; Deyang, Sichuan, China | + | + |
| 85 | A. flavus | 3.5283 | 四川成都; 土壤 Soil; Chengdu, Sichuan, China | + | + |
| 86 | A. flavus | 3.5309 | 四川都江堰; 土壤 Soil; Dujiangyan, Sichuan, China | + | + |
| 87 | A. flavus | 3.5329 | 贵州梵净山; 皮革 Leather; Mt. Fanjingshan, Guizhou, China | + | + |
| 88 | A. flavus | 3.6153 | 山东泰安; 小麦 Wheat; Tai’an, Shandong, China | - | + |
| 89 | A. flavus | 3.6304 | 广西宜山; 玉米 Corn; Yishan, Guangxi, China | - | + |
| 90 | A. flavus | 3.6307 | 吉林珲春; 亚麻 Linen; Hunchun, Jilin, China | + | + |
| 91 | A. flavus | 3.6311 | 广东广州; 空气 Air; Guangzhou, Guangdong, China | + | + |
| 92 | A. flavus | 3.6422 | 河北小五台山; 松果 Pinecore; Mt. Small Wutaishan, Hebei, China | + | + |
| 93 | A. flavus | 3.6428 | 云南大理; 霉纸 Mouldy paper; Dali, Yunnan, China | + | + |
| 94 | A. flavus | 3.6431 | 云南大理; 玉米叶 Corn leaves; Dali, Yunnan, China | + | + |
| 95 | A. flavus | 3.6434 | 云南思茅; 土壤 Soil; Simao, Yunnan, China | - | + |
| 96 | A. flavus | 14160 | 河南信阳; 土壤 Soil,; Xinyang, Henan, China | - | - |
| 97 | A. flavus | FJ17 | 福建宁德; 茶叶 Tea; Ningde, Fujian, China | + | + |
| A. arachidicola | CBS 117610T | 阿根廷; 花生叶 Arachis glabrata leaves; Argentina | |||
| A. minisclerotigenes | CBS 115635T | 阿根廷; 花生 Arachis hypogaea seeds; Argentina | |||
| A. parasiticus | CBS 100926T | 美国夏威夷; 嗜桔粉蚧 Pseudococcus calceolariae; Hawaii, USA | |||
| 3.306 | 天津; 酱曲 Soy sauce starter; Tianjin, China | ||||
| A. aflatoxiformans | CBS 143679T | 尼日利亚; 土壤 Soil; Nigeria | |||
| A. tamarii | CBS 104.13T | 分离地未知; 活性碳 Activated carbon; unknown country | |||
表1 (续)
Table 1 (continued)
| 菌株顺序号* Number | 物种 Species | 菌株 Strains | 分离地和基物 Isolation places and substrates | 产毒素 Toxin production | |
|---|---|---|---|---|---|
| AFB | CPA | ||||
| 79 | A. flavus | 3.3554 | 北京; 空气 Air; Beijing, China | - | + |
| 80 | A. flavus var. columnaris | CBS 485.65T | 日本; 黄油 Ex-type of A. flavus var. columnaris, butter; Japan | - | + |
| 81 | A. flavus | 3.4408-2 | 北京; 空气 Air; Beijing, China | + | + |
| 82 | A. flavus | 3.4410 | 美国 ATCC 28539; USA | + | + |
| 83 | A. flavus | 3.5211 | 北京 CICC 2348; Beijing, China | - | + |
| 84 | A. flavus | 3.5278 | 四川德阳; 烂水果 Rotten fruit; Deyang, Sichuan, China | + | + |
| 85 | A. flavus | 3.5283 | 四川成都; 土壤 Soil; Chengdu, Sichuan, China | + | + |
| 86 | A. flavus | 3.5309 | 四川都江堰; 土壤 Soil; Dujiangyan, Sichuan, China | + | + |
| 87 | A. flavus | 3.5329 | 贵州梵净山; 皮革 Leather; Mt. Fanjingshan, Guizhou, China | + | + |
| 88 | A. flavus | 3.6153 | 山东泰安; 小麦 Wheat; Tai’an, Shandong, China | - | + |
| 89 | A. flavus | 3.6304 | 广西宜山; 玉米 Corn; Yishan, Guangxi, China | - | + |
| 90 | A. flavus | 3.6307 | 吉林珲春; 亚麻 Linen; Hunchun, Jilin, China | + | + |
| 91 | A. flavus | 3.6311 | 广东广州; 空气 Air; Guangzhou, Guangdong, China | + | + |
| 92 | A. flavus | 3.6422 | 河北小五台山; 松果 Pinecore; Mt. Small Wutaishan, Hebei, China | + | + |
| 93 | A. flavus | 3.6428 | 云南大理; 霉纸 Mouldy paper; Dali, Yunnan, China | + | + |
| 94 | A. flavus | 3.6431 | 云南大理; 玉米叶 Corn leaves; Dali, Yunnan, China | + | + |
| 95 | A. flavus | 3.6434 | 云南思茅; 土壤 Soil; Simao, Yunnan, China | - | + |
| 96 | A. flavus | 14160 | 河南信阳; 土壤 Soil,; Xinyang, Henan, China | - | - |
| 97 | A. flavus | FJ17 | 福建宁德; 茶叶 Tea; Ningde, Fujian, China | + | + |
| A. arachidicola | CBS 117610T | 阿根廷; 花生叶 Arachis glabrata leaves; Argentina | |||
| A. minisclerotigenes | CBS 115635T | 阿根廷; 花生 Arachis hypogaea seeds; Argentina | |||
| A. parasiticus | CBS 100926T | 美国夏威夷; 嗜桔粉蚧 Pseudococcus calceolariae; Hawaii, USA | |||
| 3.306 | 天津; 酱曲 Soy sauce starter; Tianjin, China | ||||
| A. aflatoxiformans | CBS 143679T | 尼日利亚; 土壤 Soil; Nigeria | |||
| A. tamarii | CBS 104.13T | 分离地未知; 活性碳 Activated carbon; unknown country | |||
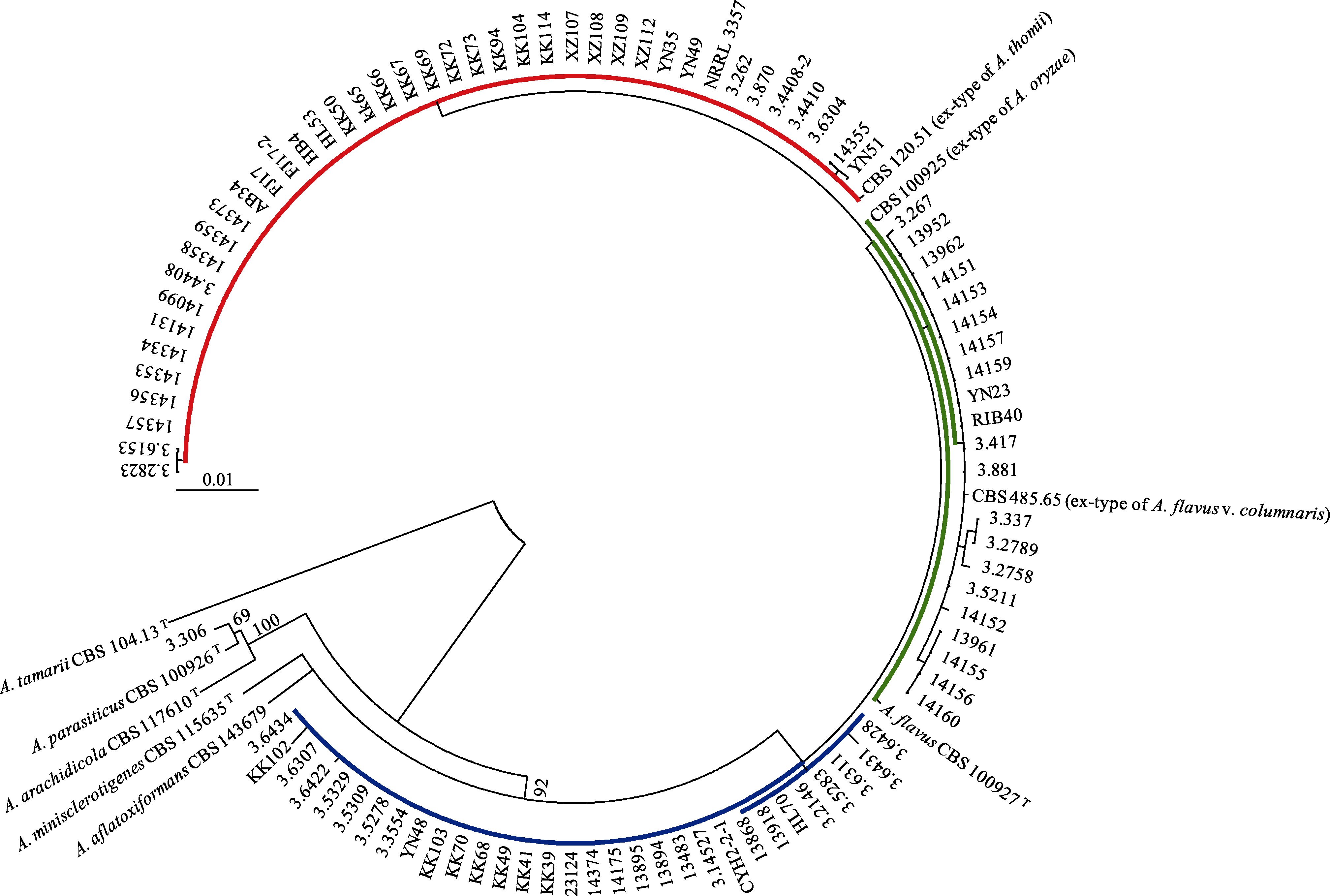
图1 97株黄曲霉及其4个近缘种的ML系统发育树。黄曲霉模式菌株CBS 100927T、黄曲霉柱头变种模式菌株CBS 485.65T、米曲霉模式菌株CBS 100925T和托姆曲霉模式菌株CBS 120.51T同在一个分支, 支持率为92%, 溜曲霉模式菌株CBS 104.13T作为外群。红、绿、蓝颜色与图3对应。
Fig. 1 The maximum likelihood phylogram of 97 Aspergillus flavus strains and its four close-related species. The ex-type of A. flavus CBS 100927T, ex-type of A. flavus var. columnaris CBS485.65T, ex-type of A. oryzae CBS 100925T, and ex-type of A. thomii CBS 120.51T are in the same clade with a 92% support, with the ex-type of A. tamari CBS 104.13T as the outgroup. The red, green and blue colours are in accordance with Fig. 3.
| K | Replicates | Mean LnP(K) | Stdev LnP(K) | Ln°(K) | |Ln?(K)| | Delta K |
|---|---|---|---|---|---|---|
| 2 | 20 | -252.900000 | 11.116323 | - | - | - |
| 3 | 20 | -153.585000 | 0.665918 | 99.315000 | 115.770000 | 173.850119 |
| 4 | 20 | -170.040000 | 4.371607 | -16.455000 | 14.975000 | 3.425514 |
| 5 | 20 | -171.520000 | 3.293790 | -1.480000 | 16.860000 | 5.118723 |
| 6 | 20 | -189.860000 | 14.895474 | -18.340000 | 402.695000 | 27.034721 |
| 7 | 20 | -610.895000 | 1,768.728466 | -421.035000 | 779.785000 | 0.440873 |
| 8 | 20 | -252.145000 | 53.166892 | 358.750000 | — | — |
表2 由Structure 2.3.4根据Delta K推导出的最佳居群数目K
Table 2 The best population number K inferred by Structure 2.3.4
| K | Replicates | Mean LnP(K) | Stdev LnP(K) | Ln°(K) | |Ln?(K)| | Delta K |
|---|---|---|---|---|---|---|
| 2 | 20 | -252.900000 | 11.116323 | - | - | - |
| 3 | 20 | -153.585000 | 0.665918 | 99.315000 | 115.770000 | 173.850119 |
| 4 | 20 | -170.040000 | 4.371607 | -16.455000 | 14.975000 | 3.425514 |
| 5 | 20 | -171.520000 | 3.293790 | -1.480000 | 16.860000 | 5.118723 |
| 6 | 20 | -189.860000 | 14.895474 | -18.340000 | 402.695000 | 27.034721 |
| 7 | 20 | -610.895000 | 1,768.728466 | -421.035000 | 779.785000 | 0.440873 |
| 8 | 20 | -252.145000 | 53.166892 | 358.750000 | — | — |
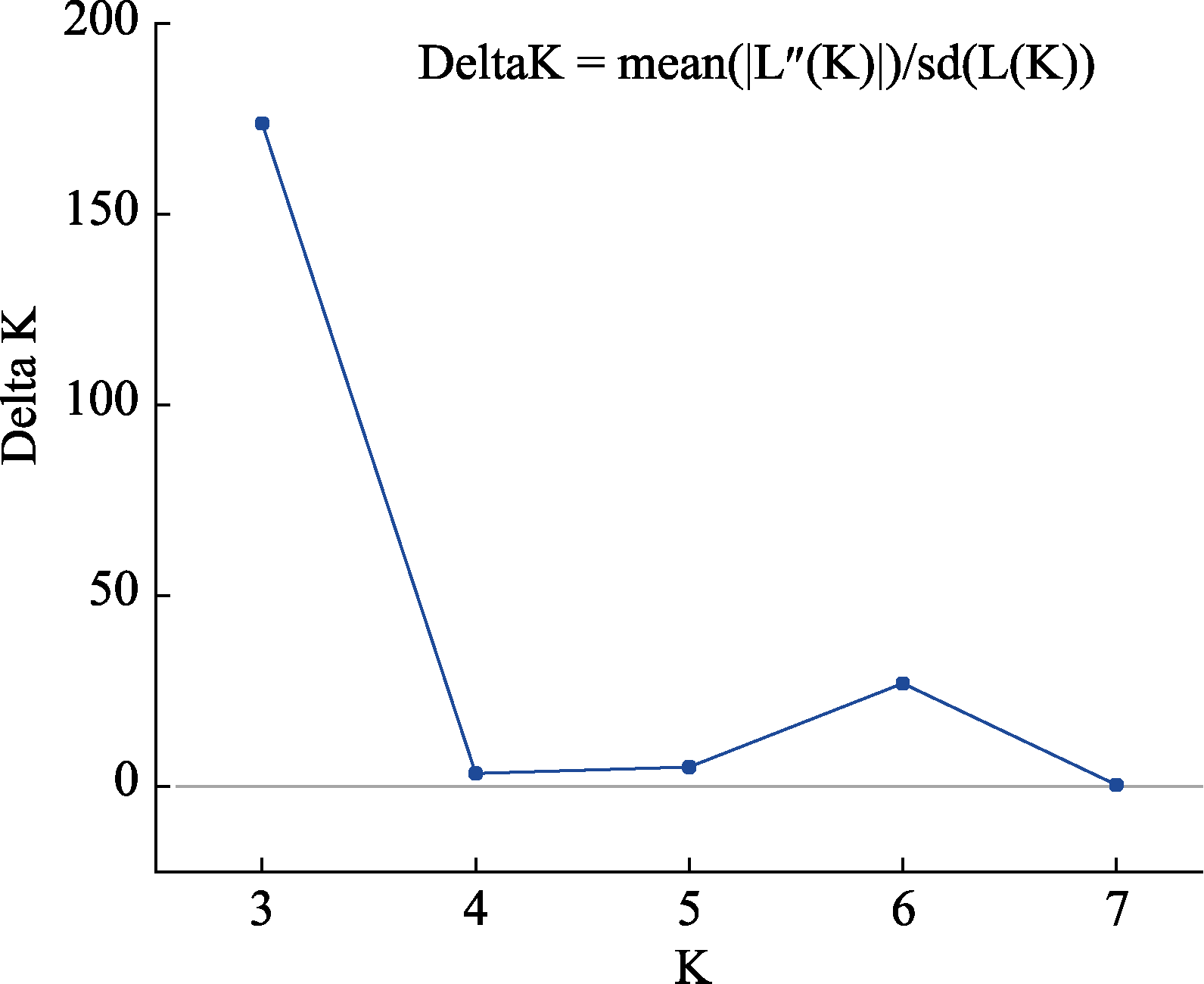
图2 Structure Harvester计算得到的最佳居群数目(delta K = 3为最佳)
Fig. 2 The best population numbers calculated by Structure Harvester (the best number is 3 for delta K)
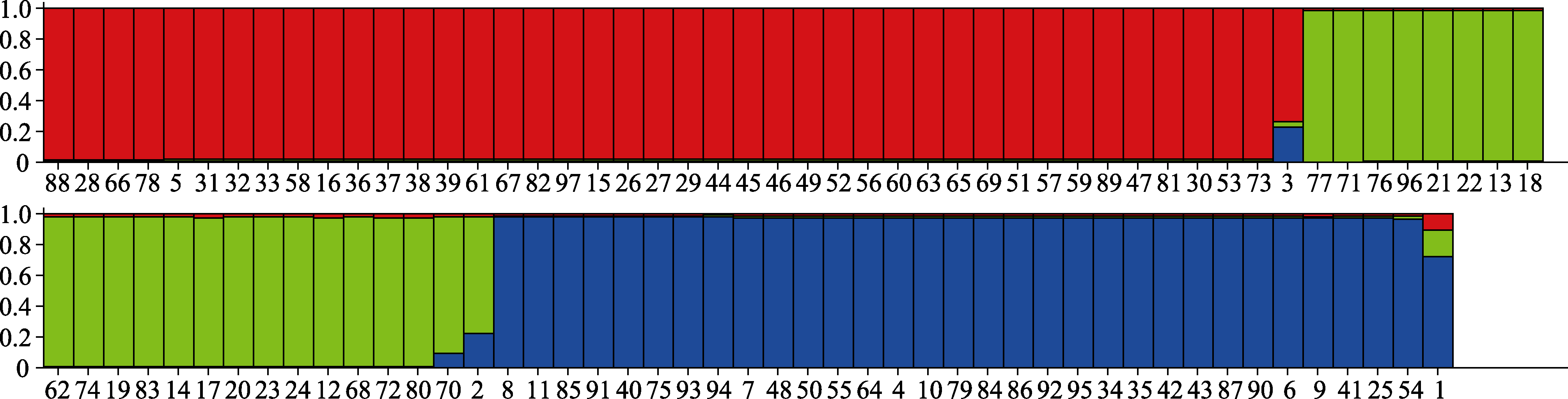
图3 97株黄曲霉由Structure Harvester计算得到3个居群。红色, 绿色和蓝色分别代表3个居群, 每一个颜色柱代表一个菌株, 菌株顺序号为颜色柱下面的数字, 与表1对应。
Fig. 3 The three populations of the 97 Aspergillus flavus isolates calculated by Structure Harvester. Red, green and blue colours stand for the three different populations, each column stands for each strain, and the number under each column is in accordance with those in Table 1.
| 1 | Anderson B, Thrane U ( 2006) Food-borne fungi in fruit and cereals and their production of mycotoxins. In: Advances in Food Mycology (eds Hocking AD, Pitt JI, Samson RA, Thrane U), pp. 137-152. Springer, Boston. |
| 2 | Barros G, Torres A, Chulze S ( 2005) Aspergillus flavus population isolated from soil of Argentina’s peanut-growing region. Sclerotia production and toxigenic profile. Journal of the Science of Food and Agriculture, 85, 2349-2353. |
| 3 | Batista PP, Santos JF, Oliveira NT, Pires APD, Motta CMS, Luna-Alves Lima EA ( 2008) Genetic characterization of Brazilian strains of Aspergillus flavus using DNA markers. Genetics and Molecular Research, 7, 706-717. |
| 4 | CAST ( Council for Agricultural Science and Technology) ( 2003) Mycotoxins: Risks in plant, animal and human systems. Task Force Report No. 139, 13-85. CAST, Ames, IA. |
| 5 | Chang PK, Ehrlich KC ( 2010) What does genetic diversity of Aspergillus flavus tell us about Aspergillus flavus? International Journal of Food Microbiology, 138, 189-199. |
| 6 | Chang PK, Ehrlich KC, Hua SS ( 2006) Cladal relatedness among Aspergillus flavus isolates and Aspergillus flavus S and L morphotype isolates. International Journal of Food Microbiology, 108, 172-177. |
| 7 | Cotty PJ ( 1997) Aflatoxin-producing potential of communities of Aspergillus section Flavi from cotton producing areas in the United States. Mycological Research, 101, 698-704. |
| 8 | Cotty PJ ( 1989) Virulence and cultural characteristics of two Aspergillus flavus strains pathogenic on cotton. Phytopathology, 79, 808-814. |
| 9 | Cotty PJ, Jaime-Garcia R ( 2007) Influence of climate on aflatoxin producing fungi and aflatoxin contamination. International Journal of Food Microbiology, 119, 109-115. |
| 10 | Ehrlich KC, Chang P-K, Yu J, Cotty PJ ( 2004) Aflatoxin biosynthesis cluster gene cypA is required for G aflatoxin formation. Applied and Environmental Microbiology, 70, 6518-6524. |
| 11 | Frisvad JC, Skouboe P, Samson RA ( 2005) Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylstergmatocystin, Aspergillus rambellii sp. nov. Systematic and Applied Microbiology, 28, 442-453. |
| 12 | Frisvad JC, Hubka V, Ezekiel CN, Hong SB, Novakova A, Chen AJ, Arzanlou M, Larsen TO, Sklenar F, Mahakarnchanakul W, Samson RA, Houbraken J ( 2019) Taxonomy of Aspergillus section Flavi and their production of aflatoxins, ochratoxins and other mycotoxins. Studies in Mycology, 93, 1-63. |
| 13 | Gao XF, Yin SA, Ji R ( 2011) Contamination of aflatoxins in peanuts from some regions in China. Chinese Journal of Public Health, 27, 541-542. |
| (in Chinese with English abstract) [ 高秀芬, 荫士安, 计融 ( 2011) 中国部分地区花生中4种黄曲霉毒素污染调查. 中国公共卫生, 27, 541-542.] | |
| 14 | Geiser DM, Dorner JW, Horn BW, Taylor JW ( 2000) The phylogenetics of mycotoxin and sclertium production in Aspergillus flavus and Aspergillus oryzae. Fungal Genetics and Biology, 31, 169-179. |
| 15 | Glass NL, Donaldson GC ( 1995) Development of primer sets designed for use with the PCR to amplify conserved genes from filamentous ascomycetes. Applied and Environmental Microbiology, 61, 1323-1330. |
| 16 | Hall TA ( 1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symposium Series, 41, 95-98. |
| 17 | Hedayati MT, Pasqualotto AC, Warn PA, Bowyer P, Denning DW ( 2007) Aspergillus flavus: Human pathogen, allergen and mycotoxin producer. Microbiology, 153, 1677-1692. |
| 18 | Horn BW, Greene RL, Dorner JW ( 1995) Effect of corn and peanut cultivation on soil populations of Aspergillus flavus and A. parasiticus in southwestern Georgia. Applied and Environmental Microbiology, 61, 2472-2475. |
| 19 | Horn BW, Dorner JW ( 1999) Regional differences in production of aflatoxin B1 and cyclopiazonic acid by soil isolates of Aspergillus flavus along a transect within the United States. Applied and Environmental Microbiology, 65, 1444-1449. |
| 20 | Horn BW ( 2003) Ecology and population biology of aflatoxingenic fungi in soil. Journal of Toxicology-Toxin Reviews, 22, 351-379. |
| 21 | Krishnan S, Manavathu EK, Chandrasekar PH , ( 2009) Aspergillus flavus: An emerging non-fumigatus Aspergillus species of significance. Mycoses, 52, 206-222. |
| 22 | Kurtzman CP, Smiley MJ, Robnett CJ, Wicklow DT ( 1986) DNA relatedness among wild and domesticated species in the Aspergillus flavus Group. Mycologi, 78, 955-959. |
| 23 | Malloch D ( 1981) Moulds Their Isolation, Cultivation and Identification. University of Toronto Press, Toronto. |
| 24 | Medina A, Gilbert MK, Mack BM, Brian GR, Rodríguez A, Bhatnagar D, Payne G, Magan N ( 2017) Interactions between water activity and temperature on the Aspergillus flavus transcriptome and aflatoxin B1 production. International Journal of Food Microbiology, 256, 36-44. |
| 25 | Orum TV, Bigelow DM, Nelson MR, Howell DR, Cotty PJ ( 1997) Spatial and temporal patterns of Aspergillus flavus strain composition and propagule density in Yuma County, Arizona, soils. Plant Diseases, 81, 911-916. |
| 26 | Paterson RRM, Lima N ( 2010) How will climate change affect mycotoxins in food? Food Research International, 43, 1902-1914. |
| 27 | Pildain MB, Frisvad JC, Vaamonde G, Cabral D, Varga J, Samson RA ( 2008) Two novel aflatoxin-producing Aspergillus species from Argentinean peanuts. International Journal of Systematic and Evolutionary Microbiology, 58, 725-735. |
| 28 | Pitt JI, Hocking AD ( 2009) Fungi and Food spoilage, 3rd edn. Springer-Science Media, London. |
| 29 | Qi ZT, Kong HZ, Sun ZM ( 1997) Flora Fungorum Sinicorum Vol. 5. Aspergillus et teleomorphi cognate. Science Press, Beijing. |
| ( In Chinese) [ 齐祖同, 孔华忠, 孙曾美( 1997) 中国真菌志第五卷: 曲霉属及其相关有性型. 科学出版社, 北京.] | |
| 30 | Raper KB, Fennell DI ( 1965) The Genus Aspergillus. Williams & Wilkins, Baltimore. |
| 31 | Saito M, Tsuruta O ( 1993) A new variety of Aspergillus flavus from tropical soil in Thailand and its aflatoxin productivity. Proceedings of the Japanese Association of Mycotoxicology, 37, 31-36. |
| 32 | Sepahvand A, Shams-Ghahfarokhi M, Allameh A, Jahanshiri Z, Jamali M, Razzaghi-Abyaneh M ( 2011) A survey on distribution and toxigenicity of Aspergillus flavus from indoor and outdoor hospital environments. Folia Microbiologica, 56, 527-534. |
| 33 | Tamura K, Peterson D, Peterson N, Stecher G, Nei M, Kumar S ( 2011) MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 8, 2731-2739. |
| 34 | Varga J, Frisvad JC, Samson RA ( 2011) Two new aflatoxin producing species, and an overview of Aspergillus section Flavi. Studies in Mycology, 69, 57-80. |
| 35 | Wang L, Zhuang WY ( 2004) Designing primer sets for amplification of partial calmodulin genes from penicillia. Mycosystema, 23, 466-473. |
| 36 | Wang L ( 2012) Four new records of Aspergillus section Usti from Shandong Province, China. Mycotaxon, 120, 373-384. |
| 37 | Wang ZG, Zhe T, Cheng SY, Cong LM ( 1993) Study of pectinase and sclerotium producing abilities of two kinds of Aspergillus flavus isolated from Zhejiang. Mycopathologia, 121, 163-168. |
| 38 | Wang HC, Huang YC, Wang J, Wang MS, Shang SH, Ye DY, Long MJ ( 2014) Fungi isolation and identification of tobacco seeds. Chinese Tobacco Science, 35(5), 84-88. |
| (in Chinese with English abstract) [ 汪汉成, 黄艳飞, 王进, 王茂胜, 商胜华, 叶定勇, 龙明锦 ( 2014) 烟草种子携带病原真菌的分离与鉴定. 中国烟草科学, 35(5), 84-88.] |
| [1] | 吴晓晴 张美惠 葛苏婷 李漫淑 宋坤 沈国春 达良俊 张健. 上海近自然林重建过程中木本植物物种多样性与地上生物量的时空动态——以闵行区生态岛为例[J]. 生物多样性, 2025, 33(5): 24444-. |
| [2] | 干靓 刘巷序 鲁雪茗 岳星. 全球生物多样性热点地区大城市的保护政策与优化方向[J]. 生物多样性, 2025, 33(5): 24529-. |
| [3] | 曾子轩 杨锐 黄越 陈路遥. 清华大学校园鸟类多样性特征与环境关联[J]. 生物多样性, 2025, 33(5): 24373-. |
| [4] | 周昊, 王茗毅, 张楚格, 肖治术, 欧阳芳. 昆虫旅馆在独栖蜂多样性保护中的现状与挑战[J]. 生物多样性, 2025, 33(5): 24472-. |
| [5] | 臧明月, 刘立, 马月, 徐徐, 胡飞龙, 卢晓强, 李佳琦, 于赐刚, 刘燕. 《昆明-蒙特利尔全球生物多样性框架》下的中国城市生物多样性保护[J]. 生物多样性, 2025, 33(5): 24482-. |
| [6] | 祝晓雨, 王晨灏, 王忠君, 张玉钧. 城市绿地生物多样性研究进展与展望[J]. 生物多样性, 2025, 33(5): 25027-. |
| [7] | 袁琳, 王思琦, 侯静轩. 大都市地区的自然留野:趋势与展望[J]. 生物多样性, 2025, 33(5): 24481-. |
| [8] | 胡敏, 李彬彬, Coraline Goron. 只绿是不够的: 一个生物多样性友好的城市公园管理框架[J]. 生物多样性, 2025, 33(5): 24483-. |
| [9] | 王欣, 鲍风宇. 基于鸟类多样性提升的南滇池国家湿地公园生态修复效果分析[J]. 生物多样性, 2025, 33(5): 24531-. |
| [10] | 明玥, 郝培尧, 谭铃千, 郑曦. 基于城市绿色高质量发展理念的中国城市生物多样性保护与提升研究[J]. 生物多样性, 2025, 33(5): 24524-. |
| [11] | 徐欢, 辛凤飞, 施宏亮, 袁琳, 薄顺奇, 赵欣怡, 邓帅涛, 潘婷婷, 余婧, 孙赛赛, 薛程. 生态修复技术集成应用对长江口北支生境与鸟类多样性提升效果评估[J]. 生物多样性, 2025, 33(5): 24478-. |
| [12] | 谢淦, 宣晶, 付其迪, 魏泽, 薛凯, 雒海瑞, 高吉喜, 李敏. 草地植物多样性无人机调查的物种智能识别模型构建[J]. 生物多样性, 2025, 33(4): 24236-. |
| [13] | 王太, 宋福俊, 张永胜, 娄忠玉, 张艳萍, 杜岩岩. 河西走廊内陆河水系鱼类多样性及资源现状[J]. 生物多样性, 2025, 33(4): 24387-. |
| [14] | 褚晓琳, 张全国. 演化速率假说的实验验证研究进展[J]. 生物多样性, 2025, 33(4): 25019-. |
| [15] | 张浩斌, 肖路, 刘艳杰. 夜间灯光对外来入侵植物和本地植物群落多样性和生长的影响[J]. 生物多样性, 2025, 33(4): 24553-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn