



Biodiv Sci ›› 2023, Vol. 31 ›› Issue (8): 23081. DOI: 10.17520/biods.2023081 cstr: 32101.14.biods.2023081
Special Issue: 美丽中国建设
• Reviews • Previous Articles Next Articles
Jiaojiao Wu1,2, Guanting Guo1,2, Dong Chen1,2, Xin Zhao1,2, Mingzhong Long3, Dengfu Wang4, Xiaona Li1,2,*( )
)
Received:2023-03-19
Accepted:2023-07-17
Online:2023-08-20
Published:2023-09-05
Contact:
*E-mail: maidoulxn413@163.com
Jiaojiao Wu, Guanting Guo, Dong Chen, Xin Zhao, Mingzhong Long, Dengfu Wang, Xiaona Li. Review of diversity and nitrogen fixation potential of bryophyte-cyanobacteria associations[J]. Biodiv Sci, 2023, 31(8): 23081.
| 生态系统 Ecosystem | 苔藓物种 Bryophyte species | 定殖率 Colonization rate (%) | 参考文献 Reference |
|---|---|---|---|
| 美国纽约 New York, America | |||
| 温带森林 Temperate forest | 地钱属一种 Marchantia sp. | 15 | Deane-Coe & Sparks, |
| 凤尾藓属一种 Fissidens sp. | 85 | ||
| 羽藓属一种 Thuidium sp. | 15 | ||
| 美国华盛顿 Washington, America | |||
| 温带草原 Temperate grassland | Kindbergia oregana | 7 (枝 Branch) | Calabria et al, |
| Racomitrium elongatum | 40 (枝 Branch) 87 (叶 Leaf) | ||
| 大拟垂枝藓 Rhytidiadelphus triquetrus | 87 (枝 Branch) 79 (叶 Leaf) | ||
| 赤茎藓 Pleurozium schreberi | 49 (枝 Branch) 33 (叶 Leaf) | ||
| 瑞典北部 Northern Sweden | |||
| 北方森林 Boreal forest | 大皱蒴藓 Aulacomnium turgidum | 34.54 (叶 Leaf) | Liu & Rousk, |
| 塔藓 Hylocomium splendens | 54.19 (叶 Leaf) | ||
| 毛青藓 Tomentypnum nitens | 19.14 (叶 Leaf) | ||
| 赤茎藓 Pleurozium schreberi | 18.19 (叶 Leaf) | ||
| 中国云南 Yunnan, China | |||
| 亚热带山地雨林 Subtropical mountain rainforest | 阿萨羽苔 Plagiochila assamica | 1.04 | Fan et al, |
| 大羽藓 Thuidium cymbifolium | 2.31 | ||
| 刀叶树平藓 Homaliodendron scalpellifolium | 3.37 | ||
| 西南树平藓 Homaliodendron montagneanu | 1.46 | ||
Table 1 Cyanobacteria colonization rates of bryophyte-cyanobacteria associations in typical ecosystems
| 生态系统 Ecosystem | 苔藓物种 Bryophyte species | 定殖率 Colonization rate (%) | 参考文献 Reference |
|---|---|---|---|
| 美国纽约 New York, America | |||
| 温带森林 Temperate forest | 地钱属一种 Marchantia sp. | 15 | Deane-Coe & Sparks, |
| 凤尾藓属一种 Fissidens sp. | 85 | ||
| 羽藓属一种 Thuidium sp. | 15 | ||
| 美国华盛顿 Washington, America | |||
| 温带草原 Temperate grassland | Kindbergia oregana | 7 (枝 Branch) | Calabria et al, |
| Racomitrium elongatum | 40 (枝 Branch) 87 (叶 Leaf) | ||
| 大拟垂枝藓 Rhytidiadelphus triquetrus | 87 (枝 Branch) 79 (叶 Leaf) | ||
| 赤茎藓 Pleurozium schreberi | 49 (枝 Branch) 33 (叶 Leaf) | ||
| 瑞典北部 Northern Sweden | |||
| 北方森林 Boreal forest | 大皱蒴藓 Aulacomnium turgidum | 34.54 (叶 Leaf) | Liu & Rousk, |
| 塔藓 Hylocomium splendens | 54.19 (叶 Leaf) | ||
| 毛青藓 Tomentypnum nitens | 19.14 (叶 Leaf) | ||
| 赤茎藓 Pleurozium schreberi | 18.19 (叶 Leaf) | ||
| 中国云南 Yunnan, China | |||
| 亚热带山地雨林 Subtropical mountain rainforest | 阿萨羽苔 Plagiochila assamica | 1.04 | Fan et al, |
| 大羽藓 Thuidium cymbifolium | 2.31 | ||
| 刀叶树平藓 Homaliodendron scalpellifolium | 3.37 | ||
| 西南树平藓 Homaliodendron montagneanu | 1.46 | ||
| 生态系统 Ecosystem | 苔藓物种 Bryophyte species | 定殖丰度 Colonization abundance | 参考文献 Reference |
|---|---|---|---|
| 瑞典斯托达伦 Stordalen, Sweden | |||
| 湿地 Wetland | 毛叶镰刀藓 Drepanocladus trichophyllu [1, 6, 9, 23] | +++ | Granhall & Selander, |
| 毛叶镰刀藓 Drepanocladus trichophyllus [2] | ++ | ||
| Sphagnum lindbergii [2, 22] | ++ | ||
| 垂枝泥炭藓 Sphagnum jensenii [22] | ++ | ||
| 岸生泥炭藓 Sphagnum riparium [1, 8] | + | ||
| 岸生泥炭藓 Sphagnum riparium [2, 6, 9, 22, 23] | +++ | ||
| 美国乔治亚大学科学农场 Plant Science Farm, University of Georgia, America | |||
| 温带草原 Temperate grassland | 真藓属一种 Bryum sp. [1, 2] | ++ | Reddy & Giddens, |
| Bryum argentum [1, 2] | +++ | ||
| Weisia controversa [1, 2] | +++ | ||
| 美国阿拉斯加州 Alaska, America | |||
| 北方森林 Boreal forest | 皱蒴藓属一种 Aulacomnium sp. | ++ | Stuart et al, |
| 曲尾藓属一种 Dicranum sp. | + | ||
| 塔藓属一种 Hylocomium sp. | +++ | ||
| 赤茎藓 Pleurozium schreberi | ++ | ||
| 金发藓属一种 Polytrichum sp. | + | ||
| 泥炭藓属一种 Sphagnum sp. | +++ | ||
| 瑞典阿比斯库科学研究站 Abisko Scientifc Research Station, Sweden | |||
| 北方森林 Boreal forest | 塔藓 Hylocomium splendens [2, 6] | + | Permin et al, |
| 赤茎藓 Pleurozium schreberi [2, 6] | + | ||
| 大皱蒴藓 Aulacomnium turgidum | ++ | Liu & Rousk, | |
| 塔藓 Hylocomium splendens | +++ | ||
| 赤茎藓 Pleurozium schreberi | + | ||
| 毛青藓 Tomentypnum nitens | ++ | ||
| 智利火地岛 Tierra del Fuego, Chile | |||
| 冰川 Glacier | Andreaea alpina [2] | + | Arróniz-Crespo et al, |
| Andreaea laxifolia [2] | + | ||
| Acroschisma wilsonii [2, 5] | ++ | ||
| Anastrophyllum involutifolium [2] | + | ||
| Blepharidophyllum densifolium [12] | + | ||
| Chiloscyphus leptanthus [2] | + | ||
| Clasmatocolea humilis [6] | + | ||
| Cryptochila grandiflora [2] | +++ | ||
| Dendroligotrichum squamosum [2] | + | ||
| Dicranoloma chilense [2] | + | ||
| Ditrichum cylindricarpum [5, 10, 11] | +++ | ||
| Racomitrium didymum [1, 2, 6] | ++ | ||
| Racomitrium laevigatum [2, 5] | +++ | ||
| 白毛砂藓 Racomitrium lanuginosum [6] | +++ | ||
| Racomitrium subcrispipilum [1, 2, 6] | +++ | ||
Table 2 Cyanobacteria abundance of bryophyte-cyanobacteria associations in typical ecosystems
| 生态系统 Ecosystem | 苔藓物种 Bryophyte species | 定殖丰度 Colonization abundance | 参考文献 Reference |
|---|---|---|---|
| 瑞典斯托达伦 Stordalen, Sweden | |||
| 湿地 Wetland | 毛叶镰刀藓 Drepanocladus trichophyllu [1, 6, 9, 23] | +++ | Granhall & Selander, |
| 毛叶镰刀藓 Drepanocladus trichophyllus [2] | ++ | ||
| Sphagnum lindbergii [2, 22] | ++ | ||
| 垂枝泥炭藓 Sphagnum jensenii [22] | ++ | ||
| 岸生泥炭藓 Sphagnum riparium [1, 8] | + | ||
| 岸生泥炭藓 Sphagnum riparium [2, 6, 9, 22, 23] | +++ | ||
| 美国乔治亚大学科学农场 Plant Science Farm, University of Georgia, America | |||
| 温带草原 Temperate grassland | 真藓属一种 Bryum sp. [1, 2] | ++ | Reddy & Giddens, |
| Bryum argentum [1, 2] | +++ | ||
| Weisia controversa [1, 2] | +++ | ||
| 美国阿拉斯加州 Alaska, America | |||
| 北方森林 Boreal forest | 皱蒴藓属一种 Aulacomnium sp. | ++ | Stuart et al, |
| 曲尾藓属一种 Dicranum sp. | + | ||
| 塔藓属一种 Hylocomium sp. | +++ | ||
| 赤茎藓 Pleurozium schreberi | ++ | ||
| 金发藓属一种 Polytrichum sp. | + | ||
| 泥炭藓属一种 Sphagnum sp. | +++ | ||
| 瑞典阿比斯库科学研究站 Abisko Scientifc Research Station, Sweden | |||
| 北方森林 Boreal forest | 塔藓 Hylocomium splendens [2, 6] | + | Permin et al, |
| 赤茎藓 Pleurozium schreberi [2, 6] | + | ||
| 大皱蒴藓 Aulacomnium turgidum | ++ | Liu & Rousk, | |
| 塔藓 Hylocomium splendens | +++ | ||
| 赤茎藓 Pleurozium schreberi | + | ||
| 毛青藓 Tomentypnum nitens | ++ | ||
| 智利火地岛 Tierra del Fuego, Chile | |||
| 冰川 Glacier | Andreaea alpina [2] | + | Arróniz-Crespo et al, |
| Andreaea laxifolia [2] | + | ||
| Acroschisma wilsonii [2, 5] | ++ | ||
| Anastrophyllum involutifolium [2] | + | ||
| Blepharidophyllum densifolium [12] | + | ||
| Chiloscyphus leptanthus [2] | + | ||
| Clasmatocolea humilis [6] | + | ||
| Cryptochila grandiflora [2] | +++ | ||
| Dendroligotrichum squamosum [2] | + | ||
| Dicranoloma chilense [2] | + | ||
| Ditrichum cylindricarpum [5, 10, 11] | +++ | ||
| Racomitrium didymum [1, 2, 6] | ++ | ||
| Racomitrium laevigatum [2, 5] | +++ | ||
| 白毛砂藓 Racomitrium lanuginosum [6] | +++ | ||
| Racomitrium subcrispipilum [1, 2, 6] | +++ | ||
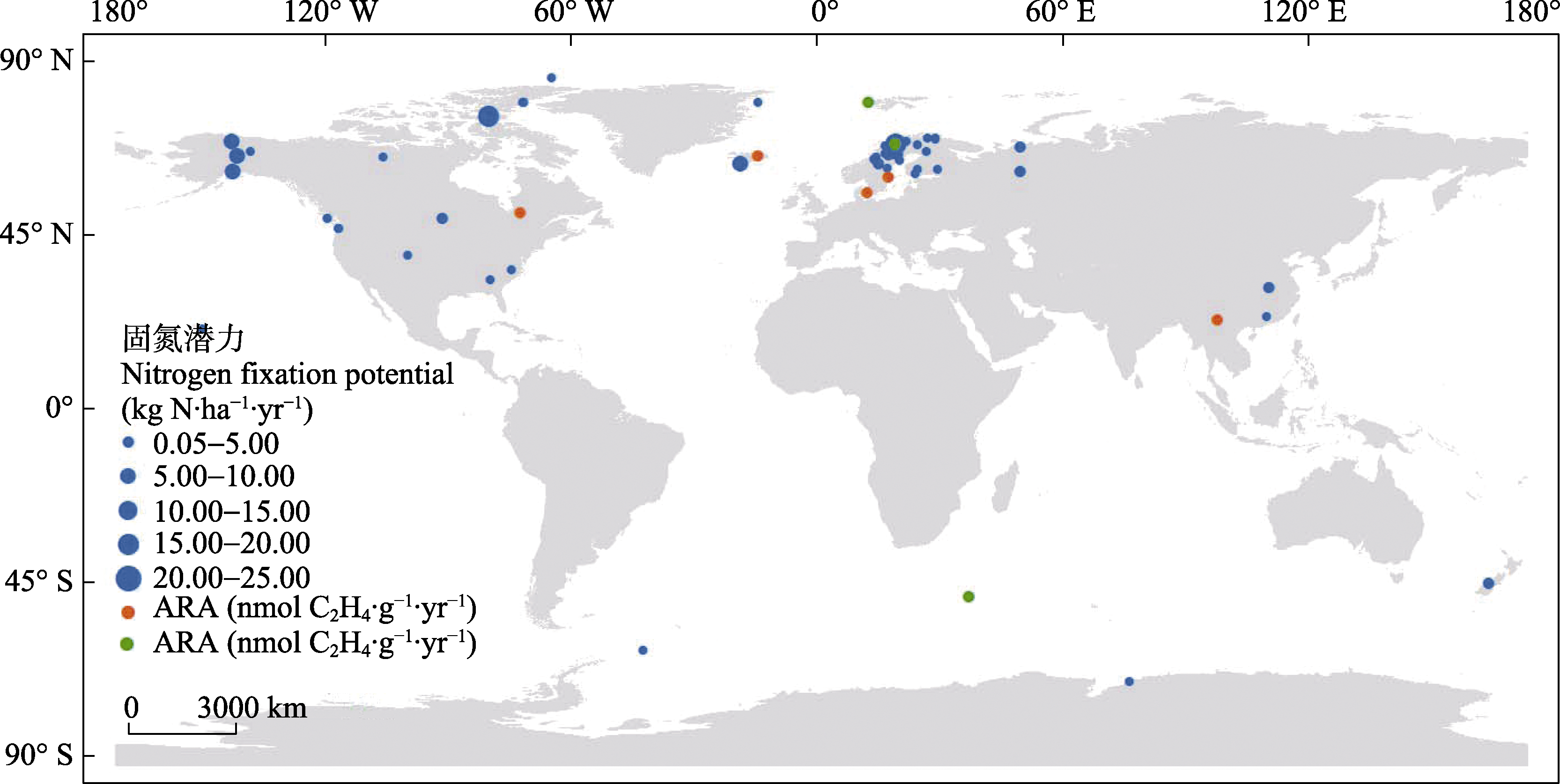
Fig. 1 Global spatial distribution of nitrogen fixation potential of bryophyte-cyanobacteria associations. The blue dots represent amount of nitrogen fixation, with size of the dot represents the level of nitrogen fixation amount. The orange/green dots only represent the locations of distribution, and represent hourly rates of nitrogen fixation calculated as wet and dry weights, respectively, regardless of the rate size. ARA, Acetylene reduction assay.
| [1] |
Aber JD (1992) Nitrogen cycling and nitrogen saturation in temperate forest ecosystems. Trends in Ecology & Evolution, 7, 220-224.
DOI URL |
| [2] |
Ackermann K, Zackrisson O, Rousk J, Jones DL, DeLuca TH (2012) N2 fixation in feather mosses is a sensitive indicator of N deposition in boreal forests. Ecosystems, 15, 986-998.
DOI URL |
| [3] | Adams DG (2002) Cyanobacteria in symbiosis. In: Cyanobacteria in Symbiosis with Hornworts and Liverworts (eds Rai AN, Bergman B, Rasmussen U), pp. 117-135. Kluwer Academic Publishers, Berlin. |
| [4] | Adams DG, Bergman B, Nierzwicki-Bauer SA, Rai AN, Schüßler A (2006) Cyanobacterial-plant symbioses. Cyanobacterial-plant symbioses. In: Symbiotic Associations, Biotechnology, Applied Microbiology (eds Dworkin M, Falkow S, Rosenberg E, Schleifer KH, Stackebrandt E), pp. 331-363. Springer, New York. |
| [5] |
Adams DG, Duggan PS (2008) Cyanobacteria-bryophyte symbioses. Journal of Experimental Botany, 59, 1047-1058.
DOI PMID |
| [6] |
Alvarenga DO, Rousk K (2021) Indirect effects of climate change inhibit N2 fixation associated with the feathermoss Hylocomium splendens in subarctic tundra. Science of the Total Environment, 795, 148676.
DOI URL |
| [7] |
Alvarenga DO, Rousk K (2022) Unraveling host-microbe interactions and ecosystem functions in moss-bacteria symbioses. Journal of Experimental Botany, 73, 4473-4486.
DOI URL |
| [8] | Arróniz-Crespo M, Pérez-Ortega S, De Los Ríos A, Green TG, Ochoa-Hueso R, Casermeiro MÁ, de la Cruz MT, Pintado A, Palacios D, Rozzi R, Tysklind N, Sancho LG (2014) Bryophyte-cyanobacteria associations during primary succession in recently Deglaciated areas of Tierra del Fuego (Chile). PLoS ONE, 9, e96081. |
| [9] |
Basilier K (1980) Fixation and uptake of nitrogen in Sphagnum blue-green algal associations. Oikos, 34, 239-242.
DOI URL |
| [10] | Bay G (2013) Symbioses Between Cyanobacterial Communities and Feather Mosses in Boreal Forests and Consequences for Dinitrogen Fixation. PhD dissertation, Faculty of Forest Sciences, Acta Universitatis Agriculturae Sueciae, Umeå. |
| [11] |
Bay G, Nahar N, Oubre M, Whitehouse MJ, Wardle DA, Zackrisson O, Nilsson MC, Rasmussen U (2013) Boreal feather mosses secrete chemical signals to gain nitrogen. New Phytologist, 200, 54-60.
DOI PMID |
| [12] | Beer C, Reichstein M, Tomelleri E, Ciais P, Jung M, Carvalhais N, Rödenbeck C, Arain MA, Baldocchi D, Bonan GB, Bondeau A, Cescatti A, Lasslop G, Lindroth A, Lomas M, Luyssaert S, Margolis H, Oleson KW, Roupsard O, Veenendaal E, Viovy N, Williams C, Woodward FI, Papale D (2010) Terrestrial gross carbon dioxide uptake: Global distribution and covariation with climate. Science, 329, 834-838. |
| [13] |
Berg A, Danielsson Å, Svensson BH (2013) Transfer of fixed-N from N2-fixing cyanobacteria associated with the moss Sphagnum riparium results in enhanced growth of the moss. Plant and Soil, 362, 271-278.
DOI URL |
| [14] |
Bills GF, Holtzman GI, Miller OK Jr (1986) Comparison of ectomycorrhizal-Basidiomycete communities in red spruce versus northern hardwood forests of West Virginia. Canadian Journal of Botany, 64, 760-768.
DOI URL |
| [15] | Bonan GB, Shugart HH (1989) Environmental factors and ecological processes in boreal forests. Annual Review of Ecology and Systematics, 20, 1-28. |
| [16] |
Bouchard R, Peñaloza-BojacáG, Toupin S, Guadalupe Y, Gudiño J, Salazar Allen N, Li FW, Villarreal AJC (2020) Contrasting bacteriome of the hornwort Leiosporoceros dussii in two nearby sites with emphasis on the hornwort-cyanobacterial symbiosis. Symbiosis, 81, 39-52.
DOI |
| [17] |
Bowman WD, Schardt JC, Schmidt SK (1996) Symbiotic N2-fixation in alpine tundra: Ecosystem input and variation in fixation rates among communities. Oecologia, 108, 345-350.
DOI URL |
| [18] |
Brandt JP, Flannigan MD, Maynard DG, Thompson ID, Volney WJA (2013) An introduction to Canada’s boreal zone: Ecosystem processes, health, sustainability, and environmental issues. Environmental Reviews, 21, 207-226.
DOI URL |
| [19] |
Calabria LM, Petersen KS, Bidwell A, Hamman ST (2020) Moss-cyanobacteria associations as a novel source of biological N2-fixation in temperate grasslands. Plant and Soil, 456, 307-321.
DOI |
| [20] | Capone DG (1993) Handbook of methods in aquatic microbial ecology. In: Determination of Nitrogenase Activity in Aquatic Samples Using the Acetylene Reduction Procedure (eds Kemp PF, Cole JJ, Sherr BF), pp. 621-631. Lewis Publishers, Boca Raton. |
| [21] |
Carrell AA, Kolton M, Glass JB, Pelletier DA, Warren MJ, Kostka JE, Iversen CM, Hanson PJ, Weston DJ (2019) Experimental warming alters the community composition, diversity, and N2fixation activity of peat moss (Sphagnum fallax) microbiomes. Global Change Biology, 25, 2993-3004.
DOI PMID |
| [22] |
Carrell AA, Veličković D, Lawrence TJ, Bowen BP, Louie KB, Carper DL, Chu RK, Mitchell HD, Orr G, Markillie LM, Jawdy SS, Grimwood J, Shaw AJ, Schmutz J, Northen TR, Anderton CR, Pelletier DA, Weston DJ (2022) Novel metabolic interactions and environmental conditions mediate the boreal peatmoss-cyanobacteria mutualism. The ISME Journal, 16, 1074-1085.
DOI |
| [23] |
Chantanaorrapint S, Peng T, Zhu RL (2014) Reappraisal of Dendroceros cucullatus (Dendrocerotaceae, Anthocerotophyta). Phytotaxa, 167, 145-149.
DOI URL |
| [24] |
Chapin DM, Bliss LC, Bledsoe LJ (1991) Environmental regulation of nitrogen fixation in a high Arctic lowland ecosystem. Canadian Journal of Botany, 69, 2744-2755.
DOI URL |
| [25] |
Cleveland CC, Townsend AR, Schimel DS, Fisher H, Howarth RW, Hedin LO, Perakis SS, Latty EF, Von Fischer JC, Elseroad A, Wasson MF (1999) Global patterns of terrestrial biological nitrogen (N2) fixation in natural ecosystems. Global Biogeochemical Cycles, 13, 623-645.
DOI URL |
| [26] | Crews TE (1999) The presence of nitrogen fixing legumes in terrestrial communities: Evolutionary vs ecological considerations. Biogeochemistry, 46, 233-246. |
| [27] |
Davey A, Marchant HJ (1983) Seasonal variation in nitrogen fixation by Nostoc commune Vaucher at the Vestfold Hills, Antarctica. Phycologia, 22, 377-385.
DOI URL |
| [28] |
Deane-Coe KK, Sparks JP (2016) Cyanobacteria associations in temperate forest bryophytes revealed by δ15N analysis. Journal of the Torrey Botanical Society, 143, 50-57.
DOI URL |
| [29] |
DeLuca TH, Zackrisson O, Nilsson MC, Sellstedt A (2002) Quantifying nitrogen-fixation in feather moss carpets of boreal forests. Nature, 419, 917-920.
DOI URL |
| [30] |
DeLuca TH, Zackrisson O, Gentili F, Sellstedt A, Nilsson MC (2007) Ecosystem controls on nitrogen fixation in boreal feather moss communities. Oecologia, 152, 121-130.
DOI PMID |
| [31] |
DeLuca TH, Zackrisson O, Gundale MJ, Nilsson MC (2008) Ecosystem feedbacks and nitrogen fixation in boreal forests. Science, 320, 1181.
DOI PMID |
| [32] |
DeLuca TH, Zackrisson O, Nilsson MC, Sun SQ, Arróniz-Crespo M (2022) Long-term fate of nitrogen fixation in Pleurozium schreberi Brid (Mit.) moss carpets in boreal forests. Applied Soil Ecology, 169, 104215.
DOI URL |
| [33] |
Deslippe JR, Egger KN, Henry GHR (2005) Impacts of warming and fertilization on nitrogen-fixing microbial communities in the Canadian High Arctic. FEMS Microbiology Ecology, 53, 41-50.
PMID |
| [34] |
Elumeeva TG, Soudzilovskaia NA, During HJ, Cornelissen JHC (2011) The importance of colony structure versus shoot morphology for the water balance of 22 subarctic bryophyte species. Journal of Vegetation Science, 22, 152-164.
DOI URL |
| [35] |
Español C, Gallardo B, Comín FA, Pino MR (2015) Constructed wetlands increase the taxonomic and functional diversity of a degraded floodplain. Aquatic Sciences, 77, 27-44.
DOI URL |
| [36] |
Fan XY, Yuan GD, Liu WY (2022) Response strategies of N-fixation by epiphytic bryophytes to water change in a subtropical montane cloud forest. Ecological Indicators, 135, 108527.
DOI URL |
| [37] |
Gauthier S, Bernier P, Kuuluvainen T, Shvidenko AZ, Schepaschenko DG (2015) Boreal forest health and global change. Science, 349, 819-822.
DOI PMID |
| [38] |
Gavazov KS, Soudzilovskaia NA, Braster M, Cornelissen JHC (2010) Isotopic analysis of cyanobacterial nitrogen fixation associated with subarctic lichen and bryophyte species. Plant and Soil, 333, 507-517.
DOI URL |
| [39] |
Gentili F, Nilsson MC, Zackrisson O, DeLuca TH, Sellstedt A (2005) Physiological and molecular diversity of feather moss associative N2-fixing cyanobacteria. Journal of Experimental Botany, 56, 3121-3127.
PMID |
| [40] |
Granhall U, Hofsten AV (1976) Nitrogenase activity in relation to intracellular organisms in Sphagnum mosses. Physiologia Plantarum, 36, 88-94.
DOI URL |
| [41] | Granhall U, Lindberg T (1978) Nitrogen fixation in some coniferous forest ecosystems. Ecological Bulletins, 26, 178-192. |
| [42] |
Granhall U, Selander H (1973) Nitrogen fixation in a subarctic mire. Oikos, 24, 8-15.
DOI URL |
| [43] |
Gundale MJ, DeLuca TH, Nordin A (2011) Bryophytes attenuate anthropogenic nitrogen inputs in boreal forests. Global Change Biology, 17, 2743-2753.
DOI URL |
| [44] |
Henriksson E, Henriksson LE, Norrman JO, Nyman PO (1987) Biological dinitrogen fixation (acetylene reduction) exhibited by blue-green algae (cyanobacteria) in association with mosses gathered on Surtsey, Iceland. Arctic and Alpine Research, 19, 432-436.
DOI URL |
| [45] |
Holland-Moritz H, Stuart J, Lewis LR, Miller S, Mack MC, McDaniel SF, Fierer N (2018) Novel bacterial lineages associated with boreal moss species. Environmental Microbiology, 20, 2625-2638.
DOI PMID |
| [46] |
Holland-Moritz H, Stuart JEM, Lewis LR, Miller SN, Mack MC, Ponciano JM, McDaniel SF, Fierer N (2021) The bacterial communities of Alaskan mosses and their contributions to N2-fixation. Microbiome, 9, 53.
DOI PMID |
| [47] |
Houle D, Bilodeau Gauthier S, Paquet S, Planas D, Warren A (2006) Identification of two genera of N2-fixing cyanobacteria growing on three feather moss species in boreal forests of Quebec, Canada. Canadian Journal of Botany, 84, 1025-1029.
DOI URL |
| [48] | Hu MY, Li YY, Ge CR, Zhang YY, Yao HY (2021) Research status and application prospects of combined nitrogen fixation in gramineous plants. Chinese Journal of Eco-Agriculture, 29, 1815-1826. (in Chinese with English abstract) |
| [ 胡梦媛, 李雅颖, 葛超荣, 张迎迎, 姚槐应 (2021) 禾本科植物联合固氮的研究现状及应用前景. 中国生态农业学报, 29, 1815-1826.] | |
| [49] |
Jean M, Mack MC, Johnstone JF (2018) Spatial and temporal variation in moss-associated dinitrogen fixation in coniferous and deciduous dominated Alaskan boreal forests. Plant Ecology, 219, 837-851.
DOI |
| [50] |
Jean ME, Cassar N, Setzer C, Bellenger JP (2012) Short-term N2fixation kinetics in a moss-associated cyanobacteria. Environmental Science & Technology, 46, 8667-8671.
DOI URL |
| [51] |
Klarenberg IJ, Keuschnig C, Russi Colmenares AJ, Warshan D, Jungblut AD, Jónsdóttir IS, Vilhelmsson O (2022) Long-term warming effects on the microbiome and nifH genea bundance of a common moss species in sub-Arctic tundra. The New Phytologist, 234, 2044-2056.
DOI URL |
| [52] |
Kostka JE, Weston DJ, Glass JB, Lilleskov EA, Shaw AJ, Turetsky MR (2016) The Sphagnum microbiome: New insights from an ancient plant lineage. New Phytologist, 211, 57-64.
DOI PMID |
| [53] |
Lagerström A, Nilsson MC, Zackrisson O, Wardle DA (2007) Ecosystem input of nitrogen through biological fixation in feather mosses during ecosystem retrogression. Functional Ecology, 21, 1027-1033.
DOI URL |
| [54] |
Lindo Z, Nilsson MC, Gundale MJ (2013) Bryophyte- cyanobacteria associations as regulators of the northern latitude carbon balance in response to global change. Global Change Biology, 19, 2022-2035.
DOI PMID |
| [55] |
Lindo Z, Whiteley JA (2011) Old trees contribute bio-available nitrogen through canopy bryophytes. Plant and Soil, 342, 141-148.
DOI URL |
| [56] | Liu S, Liu WY, Shi XM, Li S, Hu T, Song L, Wu CS (2018) Dry-hot stress significantly reduced the nitrogenase activity of epiphytic cyanolichen. Science of the Total Environment, 619/620, 630-637. |
| [57] |
Liu X, Rousk K (2022) The moss traits that rule cyanobacterial colonization. Annals of Botany, 129, 147-160.
DOI URL |
| [58] |
Liu X, Wang Z, Li XM, Rousk K, Bao WK (2020) High nitrogen resorption efficiency of forest mosses. Annals of Botany, 125, 557-563.
DOI PMID |
| [59] | Ma WZ, Liu WY, Li XJ (2009) Species composition and life forms of epiphytic bryophytes in old-growth and secondary forests in Mt. Ailao, SW China. Cryptogamie Bryologie, 30, 477-500. |
| [60] | Meeks JC (1990) Handbook of symbiotic cyanobacteria. In: Cyanobacterial-bryophyte Associations (ed. Rai AN), pp. 43-63. CRC Press, Boca Raton. |
| [61] |
Menge DNL, Hedin LO (2009) Nitrogen fixation in different biogeochemical niches along a 120000-year chronosequence in New Zealand. Ecology, 90, 2190-2201.
DOI URL |
| [62] |
Oliveira RS, Eller CB, de V Barros F, Hirota M, Brum M, Bittencourt P (2021) Linking plant hydraulics and the fast-slow continuum to understand resilience to drought in tropical ecosystems. New Phytologist, 230, 904-923.
DOI PMID |
| [63] | Patova EN, Sivkov MD, Goncharova NN, Shubina TP (2020) Associations between nitrogen-fixing cyanobacteria and Sphagnum mosses in floodplain bogs of the middle taiga (European Northeast). Theoretical and Applied Ecology, 117-123. |
| [64] |
Peng T, Zhu RL (2013) A revision of the genus Anthoceros (Anthocerotaceae, Anthocerotophyta) in China. Phytotaxa, 100, 21-35.
DOI URL |
| [65] |
Peng T, Zhu RL (2014) A revision of the genus Notothylas (Notothyladaceae, Anthocerotophyta) in China. Phytotaxa, 156, 156-164.
DOI URL |
| [66] |
Permin A, Michelsen A, Rousk K (2022) Direct and indirect effects of warming on moss abundance and associated nitrogen fixation in subarctic ecosystems. Plant and Soil, 471, 343-358.
DOI |
| [67] |
Pi CY, Liu X, Wang Z, Bao WK (2018) Bryophyte- cyanobacteria symbioses and their nitrogen fixation capacity—A review. Chinese Journal of Plant Ecology, 42, 407-418. (in Chinese with English abstract)
DOI URL |
|
[ 皮春燕, 刘鑫, 王喆, 包维楷 (2018) 苔藓-蓝藻共生体关系与固氮能力研究进展. 植物生态学报, 42, 407-418.]
DOI |
|
| [68] | Reddy GB, Giddens J (1981) Nitrogen fixation by moss-algal association in grassland. Soil Biology and Biochemistry, 13, 537-538. |
| [69] |
Renaudin M, Darnajoux R, Bellenger JP (2021) Quantification of moss-associated cyanobacteria using phycocyanin pigment extraction. Frontiers in Microbiology, 11, 611792.
DOI URL |
| [70] |
Renaudin M, Blasi C, Bradley RL, Bellenger JP (2022) New insights into the drivers of moss-associated nitrogen fixation and cyanobacterial biomass in the eastern Canadian boreal forest. Journal of Ecology, 110, 1403-1418.
DOI URL |
| [71] |
Renzaglia KS, Schuette S, Duff RJ, Ligrone R, Shaw AJ, Mishler BD, Duckett JG (2007) Bryophyte phylogeny: Advancing the molecular and morphological frontiers. The Bryologist, 110, 179-213.
DOI URL |
| [72] |
Rousk K, DeLuca TH, Rousk J (2013a) The cyanobacterial role in the resistance of feather mosses to decomposition-toward a new hypothesis. PLoS ONE, 8, e62058.
DOI URL |
| [73] | Rousk K, Jones DL, DeLuca TH (2013b) Moss-cyanobacteria associations as biogenic sources of nitrogen in boreal forest ecosystems. Frontiers in Microbiology, 4,150. |
| [74] |
Rousk K, Jones DL, DeLuca TH (2014) The resilience of nitrogen fixation in feather moss (Pleurozium schreberi)- cyanobacteria associations after a drying and rewetting cycle. Plant and Soil, 377, 159-167.
DOI URL |
| [75] |
Rousk K, Sorensen PL, Lett S, Michelsen A (2015) Across-habitat comparison of diazotroph activity in the subarctic. Microbial Ecology, 69, 778-787.
DOI PMID |
| [76] |
Rousk K, Michelsen A (2017) Ecosystem nitrogen fixation throughout the snow-free period in subarctic tundra: Effects of willow and birch litter addition and warming. Global Change Biology, 23, 1552-1563.
DOI PMID |
| [77] |
Rousk K, Degboe J, Michelsen A, Bradley R, Bellenger JP (2017a) Molybdenum and phosphorus limitation of moss-associated nitrogen fixation in boreal ecosystems. New Phytologist, 214, 97-107.
DOI URL |
| [78] |
Rousk K, Pedersen PA, Dyrnum K, Michelsen A (2017b) The interactive effects of temperature and moisture on nitrogen fixation in two temperate-arctic mosses. Theoretical and Experimental Plant Physiology, 29, 25-36.
DOI URL |
| [79] |
Rousk K, Pedersen P, PrieméA, Michelsen A (2021) Extreme freeze-thaw cycles do not affect moss-associated nitrogen fixation across a temperature gradient, but affect nutrient loss from mosses. Acta Oecologica, 113, 103796.
DOI URL |
| [80] |
Salemaa M, Lindroos AJ, Merilä P, Mäkipää R, Smolander A (2019) N2 fixation associated with the bryophyte layer is suppressed by low levels of nitrogen deposition in boreal forests. Science of the Total Environment, 653, 995-1004.
DOI |
| [81] |
Smith VR (1984) Effects of abiotic factors on acetylene reduction by cyanobacteria epiphytic on moss at a subantarctic island. Applied and Environmental Microbiology, 48, 594-600.
DOI PMID |
| [82] | Solheim B, Zielke M (2002) Cyanobacteria in symbiosis. In: Associations between Cyanobacteria and Mosses (eds Rai AN, Bergman B, Rasmussen U), pp. 137-152 Kluwer Academic Publishers, Boston. |
| [83] | Solheim B, Wiggen H, Røberg S, Spaink H (2004) Associations between Arctic cyanobacteria and mosses. Symbiosis, 37, 169-187. |
| [84] |
Sorensen PL, Michelsen A (2011) Long-term warming and litter addition affects nitrogen fixation in a subarctic heath. Global Change Biology, 17, 528-537.
DOI URL |
| [85] | Stewart WD, Fitzgerald GP, Burris RH (1967) In situ studies on N2fixation using the acetylene reduction technique. Proceedings of the National Academy of Sciences, USA, 58, 2071-2078. |
| [86] |
Stewart KJ, Lamb EG, Coxson DS, Siciliano SD (2011) Bryophyte-cyanobacterial associations as a key factor in N2-fixation across the Canadian Arctic. Plant and Soil, 344, 335-346.
DOI URL |
| [87] |
Stuart RK, Pederson ERA, Weyman PD, Weber PK, Rassmussen U, Dupont CL (2020) Bidirectional C and N transfer and a potential role for sulfur in an epiphytic diazotrophic mutualism. The ISME Journal, 14, 3068-3078.
DOI |
| [88] |
Stuart JEM, Holland-Moritz H, Lewis LR, Jean M, Miller SN, McDaniel SF, Fierer N, Ponciano JM, Mack MC (2021) Host identity as a driver of moss-associated N2 fixation rates in Alaska. Ecosystems, 24, 530-547.
DOI |
| [89] |
Tilman D, Fargione J, Wolff B, D’Antonio C, Dobson A, Howarth R, Schindler D, Schlesinger WH, Simberloff D, Swackhamer D (2001) Forecasting agriculturally driven global environmental change. Science, 292, 281-284.
DOI PMID |
| [90] |
Turetsky MR (2003) The role of bryophytes in carbon and nitrogen cycling. The Bryologist, 106, 395-409.
DOI URL |
| [91] | Vitousek PM, Menge DNL, Reed SC, Cleveland CC (2013) Biological nitrogen fixation: Rates, patterns and ecological controls in terrestrial ecosystems. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 368, 20130119. |
| [92] |
Vlassak K, Paul EA, Harris RE (1973) Assessment of biological nitrogen fixation in grassland and associated sites. Plant and Soil, 38, 637-649.
DOI URL |
| [93] |
West NJ, Adams DG (1997) Phenotypic and genotypic comparison of symbiotic and free-living cyanobacteria from a single field site. Applied and Environmental Microbiology, 63, 4479-4484.
DOI PMID |
| [94] |
Zackrisson O, DeLuca TH, Nilsson MC, Sellstedt A, Berglund LM (2004) Nitrogen fixation increases with successional age in boreal forests. Ecology, 85, 3327-3334.
DOI URL |
| [95] |
Zackrisson O, DeLuca TH, Gentili F, Sellstedt A, Jäderlund A (2009) Nitrogen fixation in mixed Hylocomium splendens moss communities. Oecologia, 160, 309-319.
DOI PMID |
| [96] |
Zhang L, Zuo Q, Li JY, Peng T (2018) A new species of Notothylas (Notothyladaceae) from Southwest China. Phytotaxa, 367, 191-195.
DOI URL |
| [97] |
Zheng MH, Zhang W, Luo YQ, Wan SQ, Fu SL, Wang SH, Liu N, Ye Q, Yan JH, Zou B, Fang CL, Ju YX, Ha DL, Zhu LW, Mo JM (2019) The inhibitory effects of nitrogen deposition on asymbiotic nitrogen fixation are divergent between a tropical and a temperate forest. Ecosystems, 22, 955-967.
DOI |
| [98] | Zhu RL (2022) Peat mosses (Sphagnum): Ecologically, economically, and scientifically important group of carbon sequestration plants. Chinese Bulletin of Botany, 57, 559-578. (in Chinese with English abstract) |
|
[ 朱瑞良 (2022) 泥炭藓: 一类具有重要生态、经济和科学价值的碳封存植物. 植物学报, 57, 559-578.]
DOI |
|
| [99] |
Zielke M, Ekker AS, Olsen RA, Spjelkavik S, Solheim B (2002) The influence of abiotic factors on biological nitrogen fixation in different types of vegetation in the high Arctic, Svalbard. Arctic, Antarctic, and Alpine Research, 34, 293-299.
DOI URL |
| [100] |
Zielke M, Solheim B, Spjelkavik S, Olsen RA (2005) Nitrogen fixation in the high arctic: Role of vegetation and environmental conditions. Arctic, Antarctic, and Alpine Research, 37, 372-378.
DOI URL |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2026 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn