



生物多样性 ›› 2020, Vol. 28 ›› Issue (11): 1311-1323. DOI: 10.17520/biods.2020409 cstr: 32101.14.biods.2020409
所属专题: 物种形成与系统进化
收稿日期:2020-10-25
接受日期:2020-12-02
出版日期:2020-11-20
发布日期:2020-12-19
通讯作者:
卢明镇
作者简介:* E-mail: mingzhen.lu@santafe.edu. ORCID: https://orcid.org/0000-0002-8707-8745基金资助:Received:2020-10-25
Accepted:2020-12-02
Online:2020-11-20
Published:2020-12-19
Contact:
Mingzhen Lu
摘要:
植物-微生物互惠共生是一种特殊的合作形式, 在整个生命和陆地生态系统的演化历史中起着至关重要的作用。在全球环境变化背景下, 植物和微生物间的互惠共生对生态系统功能的维持具有重要意义。尽管合作/互惠共生如此重要, 在生物学中却存在着对它的历史偏见与忽视。特别地, 尽管互惠共生的理论与建模发展已有较长的历史, 但不同学科分支间仍存在着多种不同的观点。本综述从两个看似对立的视角概述植物-微生物互惠共生的概念框架, 即微生物学家关心的微观机制和生态系统生态学家关注的宏观影响。宏观模型通常从一组过于简单的假设出发, 便于理论分析。但微观机制是开展定量预测的基础, 因此新一代基于过程的宏观模型需嵌入微观机制, 这对预测全球变化下的生态系统响应至关重要。此外, 希望本文也可以吸引更多学者关注合作/互惠的重要作用, 并将这一概念应用于解决其他生态学和社会学问题。
卢明镇 (2020) 植物-微生物互惠共生: 演化机制与生态功能. 生物多样性, 28, 1311-1323. DOI: 10.17520/biods.2020409.
Mingzhen Lu (2020) Plant-microbe mutualism: Evolutionary mechanisms and ecological functions. Biodiversity Science, 28, 1311-1323. DOI: 10.17520/biods.2020409.
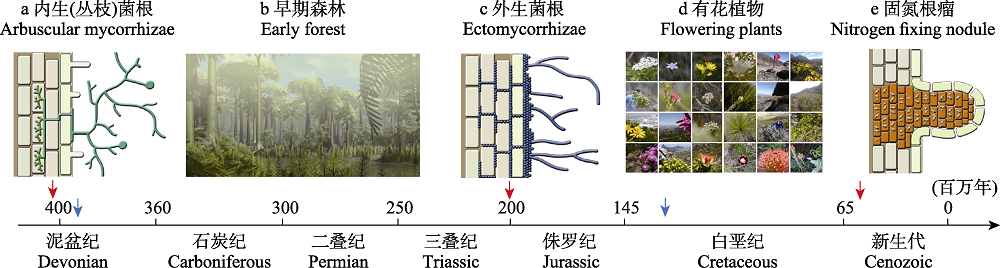
图1 陆地生态系统中植物-微生物互惠共生的演化。a. 在登陆后不久, 陆地植物与内生菌根真菌(AMF)的祖先形成了共生关系(Strullu-Derrien et al, 2014)。图中展示了单个根尖(左, 浅绿色的表皮根细胞和浅棕色的皮层细胞)和AMF菌丝是如何形成细胞内结构的。b. 早期森林出现于木本维管植物占优势的早泥盆纪(Willis & McElwain, 2014)。c. 陆地植物与外生菌根真菌(EMF) (Cairney, 2000; Martin et al, 2016)建立稳定共生关系。EMF是能够分解木质素的腐生真菌的后代, 帮助植物获取有机质中被锁住的养分。d. 受环境条件变化的选择, 早白垩纪出现了有花植物, 是植物适应性创新的一个里程碑(Willis & McElwain, 2014)。e. 植物创新的另一个里程碑事件发生在地下,白垩纪-古近纪界线不久之后(Werner et al, 2015), 植物与固氮菌形成了互惠共生关系。这些细菌(居住在橙色的细胞中)可以打开氮气的三键, 为植物供应可利用的氮。共生关系a、c、e用红色箭头指向地质时间, 地质史关键事件b、d则使用蓝色箭头。该图修改自Lu和Hedin (2019)的图1。a、c、e图作者为孙漪南, b图来自布朗大学的Andrew Lesile, d图来自作者本人。
Fig. 1 Evolution of plant-microbe mutualism in terrestrial ecosystems. a. Land plants formed associations with early ancestors of arbuscular mycorrhizal fungi (AMF) soon after the plant’s colonization of terrestrial ecosystems (Strullu-Derrien et al, 2014). The schematic illustrates how an individual root tip (left, showing epidermal root cells in light green and cortical cells in light brown) and AMF mycelial forms intracellular structure. AMF hyphae is magnitude thinner than even the thinnest plant roots, allowing them superb ability to access soil resources from the porous soil matrix. b. The early forest emerged in the early Devonian after woody vascular plants gained dominance (Willis & McElwain, 2014). c. Land plants formed associations with ectomycorrhizal fungi (EMF) (Cairney, 2000), the descendant of wood-decaying fungi, aiding plants in accessing nutrients that otherwise would be locked into organic matter. d. Selected by the changing environmental condition, flowering plants emerged during the early Cretaceous as a milestone for plants’ adaptive innovation (Willis & McElwain, 2014). e. Another milestone for plant innovation happened belowground, shortly after the Cretaceous-Paleogene boundary Werner et al (2015), with plants forming mutualistic associations with nitrogen-fixing bacteria. These bacteria (housed in these orange cells) can breakdown the triple bond of N2 gas and supply plants with plant available forms of nitrogen. The geological timings of mutualistic relationships a, c, e are indicated by red arrows, while that of geological events b, d by blue arrows. This figure is modified based on Figure 1 in Lu & Hedin (2019). Illustration in a, c, e, from Yinan Sun, b from Andrew Lesile of Brown University, and d from the author.
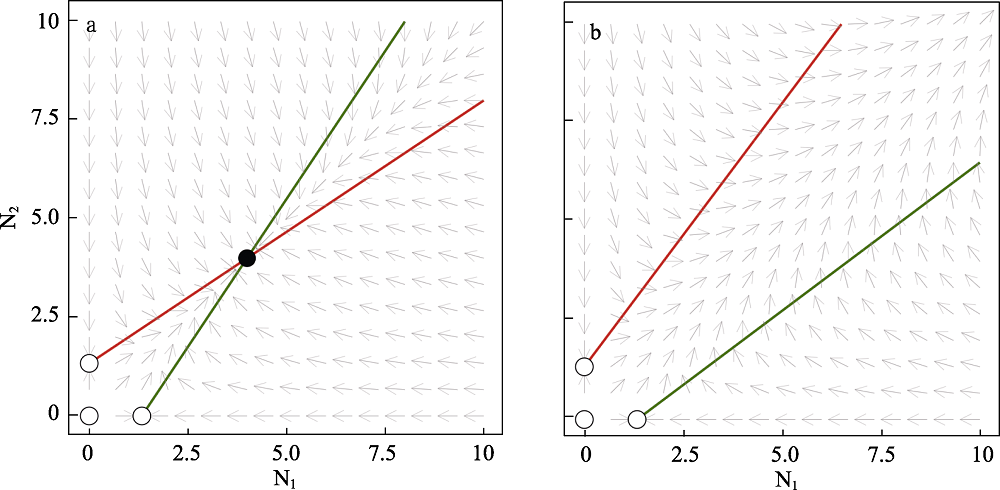
图2 互惠种群N1和N2的相平面。a. 当雅各比行列式det(J) > 0时, 相互作用导致稳定的非平凡平衡(r1 = r2 = 2, a11 = a22 = -1.5, a12 = a21 = 1)。b. 当雅各比行列式det(J) < 0时, 相互作用导致种群数量无限增长的非稳定动态(r1 = r2 = 2, a11 = a22 = -1.5, a12 = a21 = 2)。种群N1的零增长等值线用绿色, N2的零增长等值线用红色。黑色填充圆表示稳定平衡点, 白色填充圆表示非稳定和半稳定(鞍点)平衡点, 灰色箭头表示种群变化方向。绘图软件Julia 1.4.1。
Fig. 2 Phase planes of mutualistic interactions between population N1 and N2. a. The mutualistic interaction leads to stable nontrivial equilibrium when det(J) > 0 (r1 = r2 = 2, a11 = a22 = -1.5, a12 = a21 = 1). b. The mutualistic interaction leads to non-stable dynamics (infinite populations size) when det(J) < 0 (r1 = r2 = 2, a11 = a22 = -1.5, a12 = a21 = 2). The nullcline of population N1 is labeled in green and N2 in red. Black-filled circle denotes stable equilibrium while white-filled circles denote non-stable and half-stable (saddle points) equilibria. Plots are made in Julia 1.4.1.
| 研究视角 Perspective | 建模方法 Modeling approach | 优点 Strength | 弱点 Weakness |
|---|---|---|---|
| 种群生物学 Population biology | L-V方程 Lotka-Volterra equations | 熟悉, 简洁 Familiarity and simplicity | 互惠导致种群不稳定性 Infinite population due to mutualism |
| 微生物生物学 Microbial biology | 迭代囚徒困境 Iterated Prisoner’s Dilemma | 简单, 通用性 Simplicity and generality | 缺乏种群动态, 缺乏伙伴选择, 对称设置 Lack of population dynamics, lack of partner choice, and symmetric setup |
| 生物市场理论 Biological market theory | 非对称设置, 伙伴选择 Asymmetry and partner choice | 各种数学工具的混合 Lack of simplicity, mixture of tools | |
| 生态系统生态学 Ecosystem ecology | 现象学 Phenomenology | 计算效率高 Computational efficiency | 原理机制不足 Not mechanistic |
| 优化 Optimization | 概念简单 Conceptual simplicity | 任意选择的目标函数 Arbitrary goal function | |
| 自适应动态 Adaptive dynamics | 可以模拟生物对变化的响应 Can capture biological adaptation | 计算成本高, 难扩展到大尺度模型中 Computationally costly to scale up |
表1 本综述中涉及的建模方法
Table 1 A comparison of modeling approaches covered in this review
| 研究视角 Perspective | 建模方法 Modeling approach | 优点 Strength | 弱点 Weakness |
|---|---|---|---|
| 种群生物学 Population biology | L-V方程 Lotka-Volterra equations | 熟悉, 简洁 Familiarity and simplicity | 互惠导致种群不稳定性 Infinite population due to mutualism |
| 微生物生物学 Microbial biology | 迭代囚徒困境 Iterated Prisoner’s Dilemma | 简单, 通用性 Simplicity and generality | 缺乏种群动态, 缺乏伙伴选择, 对称设置 Lack of population dynamics, lack of partner choice, and symmetric setup |
| 生物市场理论 Biological market theory | 非对称设置, 伙伴选择 Asymmetry and partner choice | 各种数学工具的混合 Lack of simplicity, mixture of tools | |
| 生态系统生态学 Ecosystem ecology | 现象学 Phenomenology | 计算效率高 Computational efficiency | 原理机制不足 Not mechanistic |
| 优化 Optimization | 概念简单 Conceptual simplicity | 任意选择的目标函数 Arbitrary goal function | |
| 自适应动态 Adaptive dynamics | 可以模拟生物对变化的响应 Can capture biological adaptation | 计算成本高, 难扩展到大尺度模型中 Computationally costly to scale up |
| 玩家B (合作) Player B (Cooperate) | 玩家B (欺骗) Player B (Cheat) | |
|---|---|---|
| 玩家A (合作) Player A (Cooperate) | b-c; b-c | - c; b |
| 玩家A (欺骗) Player A (Cheat) | b; -c | 0; 0 |
表2 囚徒困境博弈特殊情况下的收益矩阵。在每一轮游戏中, 每个玩家都可以选择合作或欺骗。合作的收益为b, 成本为c, 如果双方都合作, 双方都得到b-c的回报。如果双方都不合作, 回报是零。如果一方作弊, 另一方合作, 作弊者没有支付成本c就得到了效益b, 合作者支付成本c却没有获得效益b, 博弈的纳什均衡用粗体表示。
Table 2 Payoff matrix for a special case of Prisoner’s dilemma game. Each player can choose to cooperate or cheat during each round of this game. The benefit from cooperation is b and cost of cooperation is c. If both cooperate, each get b-c. If neither cooperate, the payoff is zero. If one cheat while the other cooperate, the cheater get the benefit b without paying the cost c, and the cooperator pay the cost c without gaining the benefit b. The Nash equilibrium of this game is bolded.
| 玩家B (合作) Player B (Cooperate) | 玩家B (欺骗) Player B (Cheat) | |
|---|---|---|
| 玩家A (合作) Player A (Cooperate) | b-c; b-c | - c; b |
| 玩家A (欺骗) Player A (Cheat) | b; -c | 0; 0 |
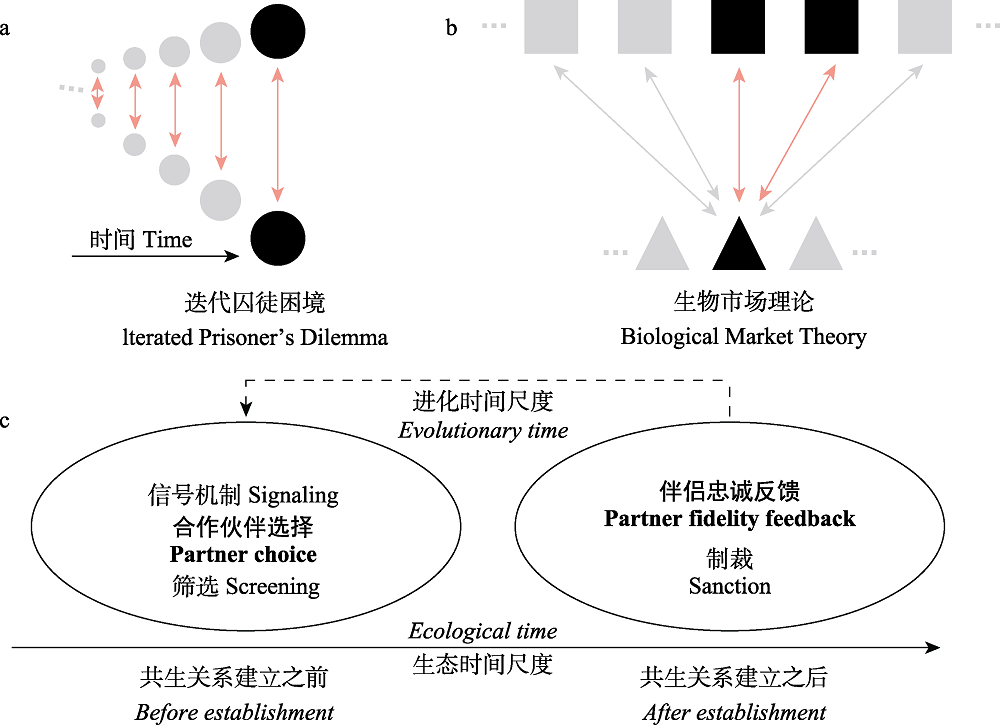
图3 迭代囚徒困境(IDP) (a)与生物市场理论(BMT) (b)的图形说明以及BMT的核心概念(c)。a. 两个玩家随着时间的推移以对称的方式重复互动, 每个玩家都能够记住最近一次的互动。玩家被标记为相同的填充点, 强调了这种方法固有的对称性(例如, 蝙蝠之间的种内合作)。b. 两组玩家通过类似于二分网络的形式进行交互作用, 其中一组中的每个玩家都可以与来自另一组的多个玩家进行交互(为了简化视觉, 这里只显示了部分交互)。这两个组在这里用不同的符号表示(正方形和三角形), 以强调这种方法内在的不对称性, 这使得它在处理特定种间的互惠互动(例如, 植物与微生物, 蜜蜂与花)时很有优势。BMT模型的最小设置用浅红色标记, 一共三个玩家, 其中一个玩家与另外两个玩家交互。c. 生物市场理论里面的最重要基本概念可以从生态时间尺度分成两类(实线时间轴): 合作伙伴选择和伴侣忠诚反馈。这两类概念所对应的生物学过程在进化时间尺度上是相连的(虚线)。
Fig. 3 Graphical illustration of Iterated Prisoner’s Dilemma (IDP) (a) vs. Biological Market Theory (BMT) (b), and core concepts used in BMT (c). a. Two players interact in a symmetric manner repeatedly over time, with each player being able to remember interactions from the immediate last time step. The players are denoted with the same filled dots, emphasizing the symmetry inherent to this approach (for example, intraspecific cooperation between individual bats). b. Two classes of players interact through a bipartite-graph alike interaction, where each player in one class can interact with multiple players from the other class (for visual simplicity, only part of the interactions are shown here). The two classes are denoted with different symbols here to emphasize the inherent asymmetry of this approach, which makes it unique in dealing with inter-specific mutualistic interaction (for example, plants vs. microbes, bees vs. flowers). The minimal setup of a BMT model is labeled in light red, where 1 player interacts with 2 other. c. The most fundamental concepts in BMT can be divided into two broad classes based on timescale (solid time arrow): partner choice vs. partner fidelity feedback. The biological processes represented by these two classes of concepts are linked over evolutionary time (dashed line).
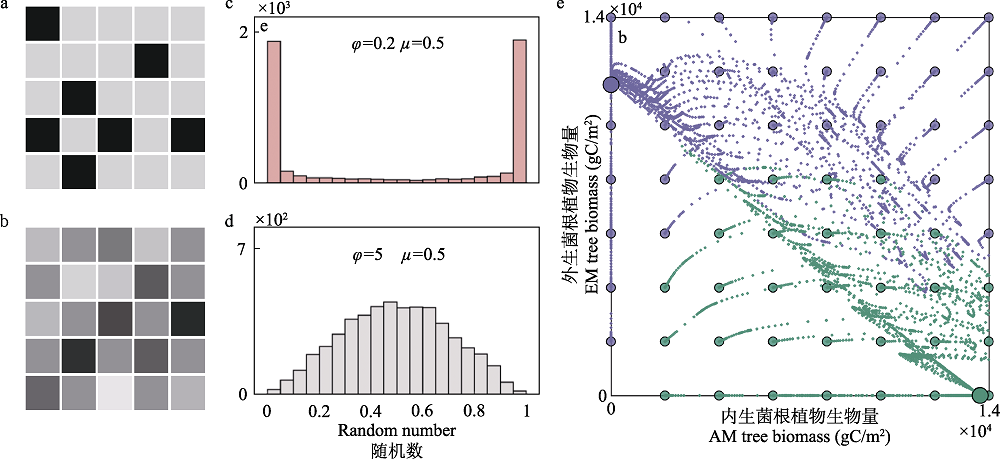
图4 双稳态植被格局及植物-微生物互惠共生的作用。a、b为植被斑块的景观图, 其中互惠共生类型A和B (在这种情况下, 丛枝菌根与外生菌根植物)的丰富度表示为色块的灰度。a所呈现的景观中, 双稳态植被状态是存在的, 一块植被要么是A主导要么是B主导, 而b则是两种互惠共生类型的随机混合。c、d互惠共生关系的分布可以用从beta分布中提取的5,000个随机数(代表5,000个景观斑块)的频率分布来说明:$f(y:\mu, phi)=cy^{\mu\varphi-1} (1-y)^{(1-\mu)\varphi-1}$ (y表示A的百分比, μ作为集中趋势, ?为离散系数)。c图所示的双峰大体可以描绘a图色块的频率分布, 而d图的单峰分布可以描绘b图色块的频率分布。e图表示A和B的初始成分(小点)随着时间的推移而分化成两种不同的稳定状态(大点, 紫色表示EMF, 绿色表示AMF)。图c、d、e摘自Lu和Hedin (2019)。
Fig. 4 Bistable vegetation patterns and the role of plant-microbe mutualism. a, b. An illustration of a landscape with patches of vegetation, where the abundance of mutualistic interaction A and B (in this case, Arbuscular mycorrhizal vs. ectomycorrhizal symbioses) is denoted by the darkness of the gray hue. a presents a landscape where bistable vegetation states is found where you either find a patch of vegetation extremely high or extremely low in one type of mutualism, whereas b has a mixture of both mutualistic type in each patch. c, d. The distribution of mutualist abundance can be illustrated using the frequency distribution of 5,000 random numbers (representing 5,000 landscape patches) drawn from a beta distribution: $f(y:\mu, phi)=cy^{\mu\varphi-1} (1-y)^{(1-\mu)\varphi-1}$ (y indicates percentage of A, μ as the central tendency, and ? as the dispersion coefficient). e. Patches that have different founding composition (small dots) of A and B will over time diverge into two alternative stable states (larger dot, EMF indicated in purple and AMF indicated in green). Panels c, d, e are reproduced from figures published in Lu & Hedin (2019).
| [1] | Averill C, Turner BL, Finzi AC (2014) Mycorrhiza-mediated competition between plants and decomposers drives soil carbon storage. Nature, 505, 543-545. |
| [2] |
Batterman SA, Hedin LO, van Breugel M, Ransijn J, Craven DJ, Hall JS (2013) Key role of symbiotic dinitrogen fixation in tropical forest secondary succession. Nature, 502, 224-227.
DOI URL PMID |
| [3] |
Bennett JA, Maherali H, Reinhart KO, Lekberg Y, Hart MM, Klironomos J (2017) Plant-soil feedbacks and mycorrhizal type influence temperate forest population dynamics. Science, 355, 181-184.
DOI URL PMID |
| [4] | Bever JD (2015) Preferential allocation, physio-evolutionary feedbacks, and the stability and environmental patterns of mutualism between plants and their root symbionts. New Phytologist, 205, 1503-1514. |
| [5] | Bowles S, Gintis H (2011) A Cooperative Species—Human Reciprocity and Its Evolution. Princeton University Press, Princeton. |
| [6] | Brzostek ER, Fisher JB, Phillips RP (2014) Modeling the carbon cost of plant nitrogen acquisition: Mycorrhizal trade-offs and multipath resistance uptake improve predictions of retranslocation. Journal of Geophysical Research: Biogeosciences, 119, 1684-1697. |
| [7] |
Cairney JWG (2000) Evolution of mycorrhiza systems. Naturwissenschaften, 87, 467-475.
DOI URL PMID |
| [8] |
Craig ME, Turner BL, Liang C, Clay K, Johnson DJ, Phillips RP (2018) Tree mycorrhizal type predicts within-site variability in the storage and distribution of soil organic matter. Global Change Biology, 24, 3317-3330.
DOI URL PMID |
| [9] | Darwin C (1859) On the Origin of Species. J. Murray, London. |
| [10] |
de Mazancourt C, Schwartz MW (2010) A resource ratio theory of cooperation. Ecology Letters, 13, 349-359.
URL PMID |
| [11] | DeAngelis DL (1992) Dynamics of Nutrient Cycling and Food Webs. Springer, Dordrecht, the Netherlands. |
| [12] | Deckmyn G, Campioli M, Muys B, Kraigher H (2011) Simulating C cycles in forest soils: Including the active role of micro-organisms in the ANAFORE forest model. Ecological Modelling, 222, 1972-1985. |
| [13] | Dercole F, Rinaldi S (2008) Analysis of Evolutionary Processes: The Adaptive Dynamics Approach and Its Applications. Princeton University Press, Princeton. |
| [14] | Diekmann O (2003) A beginner’s guide to adaptive dynamics. Banach Center Publications, 63, 1, 47-86. |
| [15] | Doebeli M, Hauert C (2005) Models of cooperation based on the Prisoner’s Dilemma and the Snowdrift game. Ecology Letters, 8, 748-766. |
| [16] | Ellsworth DS, Anderson IC, Crous KY, Cooke J, Drake JE, Gherlenda AN, Gimeno TE, MacDonald CA, Medlyn BE, Powell JR, Tjoelker MG, Reich PB (2017) Elevated CO2 does not increase eucalypt forest productivity on a low-phosphorus soil. Nature Climate Change, 7, 279-282. |
| [17] | Fisher RA, Muszala S, Verteinstein M, Lawrence P, Xu C, McDowell NG, Knox RG, Koven C, Holm J, Rogers BM, Spessa A, Lawrence D, Bonan G (2015) Taking off the training wheels: The properties of a dynamic vegetation model without climate envelopes, CLM4.5(ED). Geoscientific Model Development, 8, 3593-3619. |
| [18] | Fisher RA, Koven CD (2020) Perspectives on the future of land surface models and the challenges of representing complex terrestrial systems. Journal of Advances in Modeling Earth Systems, 12, doi: 10.1029/2018ms001453. |
| [19] |
Floudas D, Binder M, Riley R, Barry K, Blanchette RA, Henrissat B, Martínez AT, Otillar R, Spatafora JW, Yadav JS, Aerts A, Benoit I, Boyd A, Carlson A, Copeland A, Coutinho PM, de Vries RP, Ferreira P, Findley K, Foster B, Gaskell J, Glotzer D, Górecki P, Heitman J, Hesse C, Hori C, Igarashi K, Jurgens JA, Kallen N, Kersten P, Kohler A, Kües U, Kumar TKA, Kuo AL, LaButti K, Larrondo LF, Lindquist E, Ling A, Lombard V, Lucas S, Lundell T, Martin R, McLaughlin DJ, Morgenstern I, Morin E, Murat C, Nagy LG, Nolan M, Ohm RA, Patyshakuliyeva A, Rokas A, Ruiz-Dueñas FJ, Sabat G, Salamov A, Samejima M, Schmutz J, Slot JC, St John F, Stenlid J, Sun H, Sun S, Syed K, Tsang A, Wiebenga A, Young D, Pisabarro A, Eastwood DC, Martin F, Cullen D, Grigoriev IV, Hibbett DS (2012) The Paleozoic origin of enzymatic lignin decomposition reconstructed from 31 fungal genomes. Science, 336, 1715-1719.
DOI URL PMID |
| [20] | Foo E, Ferguson BJ, Reid JB (2014) Common and divergent roles of plant hormones in nodulation and arbuscular mycorrhizal symbioses. Plant Signaling & Behavior, 9, e29593. |
| [21] |
Franklin O, Harrison SP, Dewar R, Farrior CE, Brännström Å, Dieckmann U, Pietsch S, Falster D, Cramer W, Loreau M, Wang H, Mäkelä A, Rebel KT, Meron E, Schymanski SJ, Rovenskaya E, Stocker BD, Zaehle S, Manzoni S, van Oijen M, Wright IJ, Ciais P, van Bodegom PM, Peñuelas J, Hofhansl F, Terrer C, Soudzilovskaia NA, Midgley G, Prentice IC (2020) Organizing principles for vegetation dynamics. Nature Plants, 6, 444-453.
DOI URL PMID |
| [22] | Franklin O, Näsholm T, Högberg P, Högberg MN (2014) Forests trapped in nitrogen limitation―An ecological market perspective on ectomycorrhizal symbiosis. New Phytologist, 203, 657-666. |
| [23] |
Fukami T, Nakajima M (2011) Community assembly: Alternative stable states or alternative transient states? Ecology Letters, 14, 973-984.
DOI URL PMID |
| [24] | Gentry AH (1988) Changes in plant community diversity and floristic composition on environmental and geographical gradients. Annals of the Missouri Botanical Garden, 75, 1. |
| [25] | Goh BS (1979) Stability in models of mutualism. The American Naturalist, 113, 261-275. |
| [26] | Hedin LO, Brookshire ENJ, Menge DNL, Barron AR (2009) The nitrogen paradox in tropical forest ecosystems. Annual Review of Ecology, Evolution, and Systematics, 40, 613-635. |
| [27] | Hilbe C, Nowak MA, Sigmund K (2013) Evolution of extortion in iterated prisoner’s dilemma games. Proceedings of the National Academy of Sciences, USA, 110, 6913-6918. |
| [28] | Jenny H (1950) Causes of the high nitrogen and organic matter content of certain tropical forest soils. Soil Science, 69, 63-70. |
| [29] |
Jiang J, Moore JAM, Priyadarshi A, Classen AT (2017) Plant-mycorrhizal interactions mediate plant community coexistence by altering resource demand. Ecology, 98, 187-197.
URL PMID |
| [30] | Koven CD, Knox RG, Fisher RA, Chambers JQ, Christoffersen BO., Davies SJ, Detto M, Dietze MC, Faybishenko B, Holm J, Huang MY, Kovenock M, Kueppers LM, Lemieux G, Massoud E, McDowell NG, Muller-Landau HC, Needham JF, Norby RJ, Powell T, Rogers A, Serbin SP, Shuman JK, Swann ALS, Varadharajan C, Walker AP, Wright SJ, Xu CG (2020) Benchmarking and parameter sensitivity of physiological and vegetation dynamics using the Functionally Assembled Terrestrial Ecosystem Simulator (FATES) at Barro Colorado Island, Panama. Biogeosciences, 17, 3017-3044. |
| [31] | Lin GG, McCormack ML, Ma CG, Guo DL (2017) Similar below-ground carbon cycling dynamics but contrasting modes of nitrogen cycling between arbuscular mycorrhizal and ectomycorrhizal forests. New Phytologist, 213, 1440-1451. |
| [32] | Lotka AJ (1925) Elements of Physical Biology. Williams & Wilkins Company, Baltimore. |
| [33] |
Lu MZ, Hedin LO (2019) Global plant-symbiont organization and emergence of biogeochemical cycles resolved by evolution-based trait modelling. Nature Ecology & Evolution, 3, 239-250.
URL PMID |
| [34] | Luo YQ, Su B, Currie WS, Dukes JS, Finzi A, Hartwig U, Hungate B, McMurtrie RE, Oren R, Parton WJ, Pataki DE, Shaw MR, Zak DR, Field CB (2004) Progressive nitrogen limitation of ecosystem responses to rising atmospheric carbon dioxide. BioScience, 54, 731. |
| [35] |
Ma ZQ, Guo DL, Xu XL, Lu MZ, Bardgett RD, Eissenstat DM, McCormack ML, Hedin LO (2018) Evolutionary history resolves global organization of root functional traits. Nature, 555, 94-97.
DOI URL PMID |
| [36] |
Martin F, Kohler A, Murat C, Veneault-Fourrey C, Hibbett DS (2016) Unearthing the roots of ectomycorrhizal symbioses. Nature Reviews Microbiology, 14, 760-773.
DOI URL PMID |
| [37] | May RM (1974) Stability and Complexity in Model Ecosystems. Princeton University Press, Princeton. |
| [38] | Menge DNL, Levin SA, Hedin LO (2008) Evolutionary tradeoffs can select against nitrogen fixation and thereby maintain nitrogen limitation. Proceedings of the National Academy of Sciences, USA, 105, 1573-1578. |
| [39] | Menge DNL, Pacala SW, Hedin LO (2009) Emergence and maintenance of nutrient limitation over multiple timescales in terrestrial ecosystems. The American Naturalist, 173, 164-175. |
| [40] |
Menge DNL, Wolf AA, Funk JL (2015) Diversity of nitrogen fixation strategies in Mediterranean legumes. Nature Plants, 1, 15064.
URL PMID |
| [41] | Meyer A, Grote R, Polle A, Butterbach-Bahl K (2010) Simulating mycorrhiza contribution to forest C- and N cycling―The MYCOFON model. Plant and Soil, 327, 493-517. |
| [42] |
Moeller HV, Neubert MG (2016) Multiple friends with benefits: An optimal mutualist management strategy? The American Naturalist, 187, E1-E12.
URL PMID |
| [43] | Montesinos-Navarro A, Segarra-Moragues JG, Valiente-Banuet A, Verdú M (2012) The network structure of plant-arbuscular mycorrhizal fungi. New Phytologist, 194, 536-547. |
| [44] | Neuhauser C, Fargione JE (2004) A mutualism-parasitism continuum model and its application to plant-mycorrhizae interactions. Ecological Modelling, 177, 337-352. |
| [45] |
Noë R, Kiers ET (2018) Mycorrhizal markets, firms, and co-ops. Trends in Ecology & Evolution, 33, 777-789.
URL PMID |
| [46] | Noë R (1990) A veto game played by baboons: A challenge to the use of the Prisoner’s Dilemma as a paradigm for reciprocity and cooperation. Animal Behaviour, 39, 78-90. |
| [47] | Noë R, Hammerstein P (1994) Biological markets: Supply and demand determine the effect of partner choice in cooperation, mutualism and mating. Behavioral Ecology and Sociobiology, 35, 1-11. |
| [48] |
Orwin KH, Kirschbaum MUF, St John MG, Dickie IA (2011) Organic nutrient uptake by mycorrhizal fungi enhances ecosystem carbon storage: A model-based assessment. Ecology Letters, 14, 493-502.
DOI URL PMID |
| [49] | Peh KSH, Lewis SL, Lloyd J (2011) Mechanisms of monodominance in diverse tropical tree-dominated systems. Journal of Ecology, 99, 891-898. |
| [50] | Read DJ (1991) Mycorrhizas in ecosystems. Experientia, 47, 376-391. |
| [51] | Read DJ, Perez-Moreno J (2003) Mycorrhizas and nutrient cycling in ecosystems―A journey towards relevance? New Phytologist, 157, 475-492. |
| [52] |
Santos FC, Pacheco JM (2005) Scale-free networks provide a unifying framework for the emergence of cooperation. Physical Review Letters, 95, 098104.
URL PMID |
| [53] |
Schoener TW (1976) Alternatives to Lotka-Volterra competition: Models of intermediate complexity. Theoretical Population Biology, 10, 309-333.
URL PMID |
| [54] |
Simms EL, Taylor DL (2002) Partner choice in nitrogen-fixation mutualisms of legumes and rhizobia. Integrative and Comparative Biology, 42, 369-380.
DOI URL PMID |
| [55] | Strullu-Derrien C, Kenrick P, Pressel S, Duckett JG, Rioult JP, Strullu DG (2014) Fungal associations in Horneophyton ligneri from the Rhynie Chert (c. 407 million year old) closely resemble those in extant lower land plants: Novel insights into ancestral plant-fungus symbioses. New Phytologist, 203, 964-979. |
| [56] | Sulman BN, Shevliakova E, Brzostek ER, Kivlin SN, Malyshev S, Menge DNL, Zhang X (2019) Diverse mycorrhizal associations enhance terrestrial C storage in a global model. Global Biogeochemical Cycles, 33, 501-523. |
| [57] |
Taylor BN, Chazdon RL, Menge DNL (2019) Successional dynamics of nitrogen fixation and forest growth in regenerating Costa Rican rainforests. Ecology, 100, e02637.
DOI URL PMID |
| [58] | Tedersoo L, Bahram M, Zobel M (2020) How mycorrhizal associations drive plant population and community biology. Science, 367, eaba1223. |
| [59] | Terrer C, Vicca S, Hungate BA, Phillips RP, Prentice IC (2016) Mycorrhizal association as a primary control of the CO2 fertilization effect. Science, 353, 72-74. |
| [60] |
Torti SD, Coley PD, Kursar TA (2001) Causes and consequences of monodominance in tropical lowland forests. The American Naturalist, 157, 141.
DOI URL PMID |
| [61] | Trivers RL (1971) The Evolution of Reciprocal Altruism. The Quarterly Review of Biology, 46, 35-57. |
| [62] | Vitousek PM (2004) Nutrient Cycling and Limitation. Princeton University Press, Princeton. |
| [63] | Vitousek PM, Cassman K, Cleveland C, Crews T, Field CB, Grimm NB, Howarth RW, Marino R, Martinelli L, Rastetter EB, Sprent JI (2002) Towards an ecological understanding of biological nitrogen fixation. Biogeochemistry, 57/58, 1-45. |
| [64] | Vitousek PM, Field CB (1999) Ecosystem constraints to symbiotic nitrogen fixers: A simple model and its implications. Biogeochemistry, 46, 179-202. |
| [65] |
Weng ES, Farrior CE, Dybzinski R, Pacala SW (2017) Predicting vegetation type through physiological and environmental interactions with leaf traits: Evergreen and deciduous forests in an earth system modeling framework. Global Change Biology, 23, 2482-2498.
DOI URL PMID |
| [66] |
Weng ES, Dybzinski R, Farrior CE, Pacala SW (2019) Competition alters predicted forest carbon cycle responses to nitrogen availability and elevated CO2: Simulations using an explicitly competitive, game-theoretic vegetation demographic model. Biogeosciences, 16, 4577-4599.
DOI URL |
| [67] | Werner GDA, Cornwell WK, Cornelissen JHC, Kiers ET (2015) Evolutionary signals of symbiotic persistence in the legume-rhizobia mutualism. Proceedings of the National Academy of Sciences, USA, 112, 10262-10269. |
| [68] | Willis K, McElwain J (2014) The Evolution of Plants. Oxford University Press, Oxford. |
| [69] | Wilson DS (1975) A theory of group selection. Proceedings of the National Academy of Sciences, USA, 72, 143-146. |
| [70] |
Wilson DS, Sober E (1994) Reintroducing group selection to the human behavioral sciences. Behavioral and Brain Sciences, 17, 585-608.
DOI URL |
| [71] | Wilson JB, Agnew ADQ (1992) Positive-feedback switches in plant communities. Advances in Ecological Research, 23, 263-336. |
| [72] | Wipf D, Krajinski F, Tuinen D, Recorbet G, Courty PE (2019) Trading on the arbuscular mycorrhiza market: From arbuscules to common mycorrhizal networks. New Phytologist, 223, 1127-1142. |
| [73] |
Zheng MH, Chen H, Li DJ, Luo YQ, Mo JM (2020) Substrate stoichiometry determines nitrogen fixation throughout succession in southern Chinese forests. Ecology Letters, 23, 336-347.
URL PMID |
| [1] | 李沫潼, 何拓, 李薇, 廖菁, 曾岩. 从CITES的术语看野生动植物国际贸易监管规则[J]. 生物多样性, 2025, 33(4): 24545-. |
| [2] | 付梦娣, 朱彦鹏, 任月恒, 李爽, 秦乐, 谢正君, 王清春, 张立博. 新疆野生动物通道空间布局优化[J]. 生物多样性, 2025, 33(3): 24346-. |
| [3] | 龚翠凤, 韦伟, 罗概, 韩一敏, 吴鹏程, 何梦楠, 闵清悦, 付强, 陈鹏. 大熊猫国家公园崇州片区有蹄类动物空间分布及共存关系[J]. 生物多样性, 2025, 33(3): 24260-. |
| [4] | 谷际岐, 陈建平, 赖江山. 大语言模型在生物多样性研究中的应用[J]. 生物多样性, 2024, 32(9): 24258-. |
| [5] | 韩思成, 陆道炜, 韩宇辰, 栗若寒, 杨晶, 孙戈, 杨陆, 钱俊伟, 方翔, 罗述金. 北京近郊浅山地区的野生豹猫分布及环境影响因素[J]. 生物多样性, 2024, 32(8): 24138-. |
| [6] | 白皓天, 余上, 潘新园, 凌嘉乐, 吴娟, 谢恺琪, 刘阳, 陈学业. AI辅助识别的鸟类被动声学监测在城市湿地公园中的应用[J]. 生物多样性, 2024, 32(8): 24188-. |
| [7] | 王艳丽, 张英, 戚春林, 张昌达, 史佑海, 杜彦君, 丁琼. 海南热带雨林国家公园生物多样性热点与保护空缺区域识别: 基于大型真菌与植物视角[J]. 生物多样性, 2024, 32(7): 24081-. |
| [8] | 巴苏艳, 赵春艳, 刘媛, 方强. 通过虫体花粉识别构建植物‒传粉者网络: 人工模型与AI模型高度一致[J]. 生物多样性, 2024, 32(6): 24088-. |
| [9] | 董云伟, 鲍梦幻, 程娇, 陈义永, 杜建国, 高养春, 胡利莎, 李心诚, 刘春龙, 秦耿, 孙进, 王信, 杨光, 张崇良, 张雄, 张宇洋, 张志新, 战爱斌, 贺强, 孙军, 陈彬, 沙忠利, 林强. 中国海洋生物地理学研究进展和热点: 物种分布模型及其应用[J]. 生物多样性, 2024, 32(5): 23453-. |
| [10] | 徐伟强, 苏强. 分形模型与一般性物种多度分布关系的检验解析:以贝类和昆虫群落为例[J]. 生物多样性, 2024, 32(4): 23410-. |
| [11] | 刘荆州, 钱易鑫, 张燕雪丹, 崔凤. 基于潜在迪利克雷分布(LDA)模型的旗舰物种范式研究进展与启示[J]. 生物多样性, 2024, 32(4): 23439-. |
| [12] | 曲锐, 左振君, 王有鑫, 张良键, 吴志刚, 乔秀娟, 王忠. 基于元素组的生物地球化学生态位及其在不同生态系统中的应用[J]. 生物多样性, 2024, 32(4): 23378-. |
| [13] | 王斌, 钟艺倩, 杨美雪, 吴淼锐, 王艳萍, 陆芳, 陶旺兰, 李健星, 赵弘明, 刘晟源, 向悟生, 李先琨. 喀斯特季节性雨林优势树种叶片非结构性碳水化合物空间变异及生态驱动因素[J]. 生物多样性, 2024, 32(12): 24325-. |
| [14] | 杜宇晨, 刘蓓萌, 陈俊峰, 王浩, 谢屹. 基于结构方程模型的农户保护意愿影响因素分析: 以东北虎豹国家公园珲春片区为例[J]. 生物多样性, 2024, 32(1): 23155-. |
| [15] | 耿云, 寇一祎, 范新卓, 徐姝瑶, 丛丽, 张玉钧. 基于卡诺模型的大熊猫国家公园自然教育需求研究[J]. 生物多样性, 2024, 32(1): 23101-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
