



生物多样性 ›› 2017, Vol. 25 ›› Issue (5): 549-560. DOI: 10.17520/biods.2017045 cstr: 32101.14.biods.2017045
• 研究报告: 动物多样性 • 上一篇
张雪1, 王玉蕊1, 樊阳波1,2, 罗晓甜1, 胡晓钟1, 高凤1,*( )
)
收稿日期:2017-02-18
接受日期:2017-04-17
出版日期:2017-05-20
发布日期:2017-06-06
通讯作者:
高凤
基金资助:
Xue Zhang1, Yurui Wang1, Yangbo Fan1,2, Xiaotian Luo1, Xiaozhong Hu1, Feng Gao1,*( )
)
Received:2017-02-18
Accepted:2017-04-17
Online:2017-05-20
Published:2017-06-06
Contact:
Gao Feng
摘要:
游仆类是纤毛虫中进化最为复杂和高等的一大类群, 为了进一步探索和完善游仆类的多样性, 本研究利用活体观察、蛋白银和银浸法染色技术对采自青岛小西湖的小腔游仆虫(Euplotes aediculatus)的形态学及细胞发生学进行了详尽的研究, 并在完整的形态学及发生学研究基础上, 测定了小腔游仆虫的核糖体小亚基基因(SSU rDNA)序列, 通过序列比较和分子系统树构建等方法, 对小腔游仆虫的系统地位进行了分析。结果表明: 本种鉴别特征为9根额腹棘毛, 5根横棘毛, 2根缘棘毛, 2根尾棘毛, 8列背触毛, double-eurystomus型银线系。发生学特征包括: (1)后仔虫口原基在表皮下独立发生, 前仔虫完全继承老口围带; (2)额-腹-横棘毛原基从左向右按照3:3:3:2:2的模式形成额腹棘毛和横棘毛; (3)前后仔虫最左侧额腹棘毛分别由独立产生的原基形成; (4)缘棘毛原基独立发生; (5)初级背触毛原基来自虫体中部老结构的反分化; (6)前后仔虫尾棘毛分别来自最右侧2列背触毛原基和老背触毛列末端; 这些特征显示出游仆虫属个体发生模式的高度保守性。分子系统分析与形态学数据一致, 即游仆虫属为单元发生, 且小腔游仆虫与艾美游仆虫(Euplotes amieti)、阔口游仆虫(E. eurystomus)和伍氏游仆虫(E. woodruffi)聚在一起。
张雪, 王玉蕊, 樊阳波, 罗晓甜, 胡晓钟, 高凤 (2017) 小腔游仆虫形态学、个体发育与分子系统学研究. 生物多样性, 25, 549-560. DOI: 10.17520/biods.2017045.
Xue Zhang, Yurui Wang, Yangbo Fan, Xiaotian Luo, Xiaozhong Hu, Feng Gao (2017) Morphology, ontogeny and molecular phylogeny of Euplotes aediculatus Pierson, 1943 (Ciliophora, Euplotida). Biodiversity Science, 25, 549-560. DOI: 10.17520/biods.2017045.
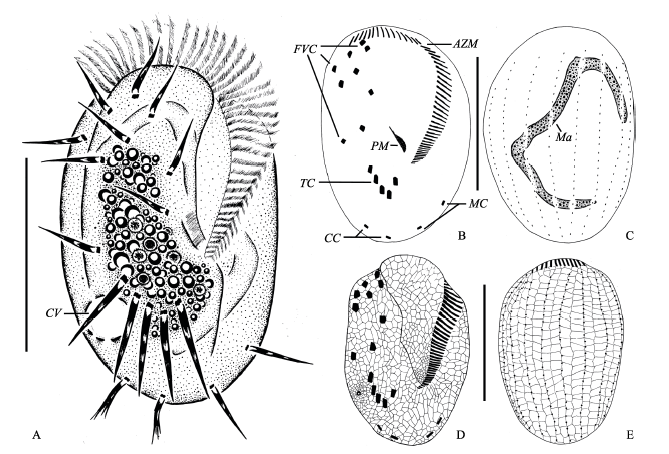
图1 小腔游仆虫的活体、蛋白银染色及银浸法染色的形态。A: 典型个体腹面观; B、C: 纤毛图式腹面(B)和背面(C)观; D、E: 腹面(D)和背面(E)银线系。AZM: 口围带; CC: 尾棘毛; CV: 伸缩泡; FVC: 额腹棘毛; Ma: 大核; MC: 缘棘毛; PM: 口侧膜; TC: 横棘毛。比例尺: 100 µm。
Fig. 1 Morphology of Euplotes aediculatus in vivo, after protargol and silver nitrate impregnation. A, Ventral view of a representative individual; B and C, Ventral (B) and dorsal (C) view of the infraciliature and nuclear apparatus; D and E, Silverline system on ventral (D) and dorsal side (E); AZM, Adoral zone of membranelles; CC, Caudal cirri; CV, Contractile vacuole; FVC, Frontoventral cirri; Ma, Macronucleus; MC, Marginal cirri; PM, Paroral membrane; TC, Transverse cirri. Scale bar = 100 µm.
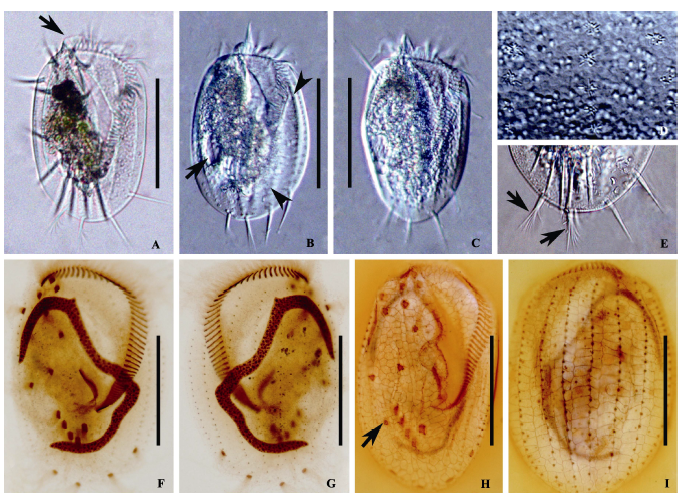
图2 小腔游仆虫的形态结构。A-E: 小腔游仆虫的活体照片; F, G: 蛋白银染色照片; H, I: 银浸法染色照片。A: 典型个体腹面观, 箭头示领状结构; B, C: 不同个体腹面观示不同体型, B图箭头指示伸缩泡, 无尾箭头指示背面肋突; D: 示虫体背面围绕背触毛的皮层颗粒; E: 虫体后部腹面观, 示缘棘毛和分叉的尾棘毛; F, G: 纤毛图式腹面(F)和背面(G)观; H, I: 腹面(H)和背面(I)银线系, H图箭头指示伸缩泡。比例尺: 100 µm。
Fig. 2 Morphology of Euplotes aediculatus. A-E, Photomicrographs in vivo; F and G, Photomicrographs after protargol; H and I, Photomicrographs after silver nitrate impregnation. A, Ventral view of a representative individual, arrow points to collar; B and C, Ventral views, showing different body shapes, arrow in picture B points to contractile vacuole, arrowheads in picture B point to ribs in the back; D, Dorsal view, showing the cortical granules arranged around the dorsal cilia; E, Posterior portion of an individual, showing the caudal cirri (arrows) and the left marginal cirri; F and G, Ventral (F) and dorsal (G) view of the infraciliature and nuclear apparatus; H and I, Silverline system on ventral (H) and dorsal side (I), arrow in picture H points to contractile vacuole. Scale bar = 100 µm.
| 特征 Character | 最小值 Minimum | 最大值 Maximum | 平均值 Mean | 中值 Median | 标准差 Standard deviation | 变异系数 Coefficient of variation | 个体数 Number of cells |
|---|---|---|---|---|---|---|---|
| 体长 Length of body (μm) | 115 | 142 | 128.3 | 129 | 7.25 | 5.7 | 20 |
| 体宽 Width of body (μm) | 76 | 101 | 92.2 | 93.5 | 6.28 | 6.8 | 20 |
| 口区长 Length of adoral zone (μm) | 79 | 98 | 86.3 | 86.5 | 4.74 | 5.5 | 20 |
| 口围带小膜数目 Number of adoral membranelles | 47 | 55 | 51.1 | 51.5 | 1.90 | 3.7 | 20 |
| 额腹棘毛数目 Number of frontoventral cirri | 9 | 9 | 9 | 9.0 | 0 | 0 | 19 |
| 横棘毛数目 Number of transverse cirri | 5 | 5 | 5.0 | 5.0 | 0 | 0 | 20 |
| 缘棘毛数目 Number of marginal cirri | 2 | 2 | 2.0 | 2.0 | 0 | 0 | 20 |
| 尾棘毛数目 Number of caudal cirri | 2 | 2 | 2.0 | 2.0 | 0 | 0 | 20 |
| 背触毛列数 Number of dorsal kineties | 8 | 8 | 8.0 | 8.0 | 0 | 0 | 20 |
| 中央背触毛列毛基体数目 Number of dikinetids in mid-dorsal kinety | 21 | 26 | 21.9 | 21.0 | 1.36 | 6.2 | 20 |
| 最左侧背触毛列毛基体数目 Number of dikinetids in the leftmost dorsal kinety | 15 | 23 | 18.5 | 18.0 | 1.73 | 9.4 | 20 |
表1 小腔游仆虫青岛小西湖种群的形态学数据。所有数据均来自蛋白银染色制片。
Table 1 Morphometric characterizations of Euplotes aediculatus from Small West Lake of Qingdao based on protargol-stained specimens
| 特征 Character | 最小值 Minimum | 最大值 Maximum | 平均值 Mean | 中值 Median | 标准差 Standard deviation | 变异系数 Coefficient of variation | 个体数 Number of cells |
|---|---|---|---|---|---|---|---|
| 体长 Length of body (μm) | 115 | 142 | 128.3 | 129 | 7.25 | 5.7 | 20 |
| 体宽 Width of body (μm) | 76 | 101 | 92.2 | 93.5 | 6.28 | 6.8 | 20 |
| 口区长 Length of adoral zone (μm) | 79 | 98 | 86.3 | 86.5 | 4.74 | 5.5 | 20 |
| 口围带小膜数目 Number of adoral membranelles | 47 | 55 | 51.1 | 51.5 | 1.90 | 3.7 | 20 |
| 额腹棘毛数目 Number of frontoventral cirri | 9 | 9 | 9 | 9.0 | 0 | 0 | 19 |
| 横棘毛数目 Number of transverse cirri | 5 | 5 | 5.0 | 5.0 | 0 | 0 | 20 |
| 缘棘毛数目 Number of marginal cirri | 2 | 2 | 2.0 | 2.0 | 0 | 0 | 20 |
| 尾棘毛数目 Number of caudal cirri | 2 | 2 | 2.0 | 2.0 | 0 | 0 | 20 |
| 背触毛列数 Number of dorsal kineties | 8 | 8 | 8.0 | 8.0 | 0 | 0 | 20 |
| 中央背触毛列毛基体数目 Number of dikinetids in mid-dorsal kinety | 21 | 26 | 21.9 | 21.0 | 1.36 | 6.2 | 20 |
| 最左侧背触毛列毛基体数目 Number of dikinetids in the leftmost dorsal kinety | 15 | 23 | 18.5 | 18.0 | 1.73 | 9.4 | 20 |
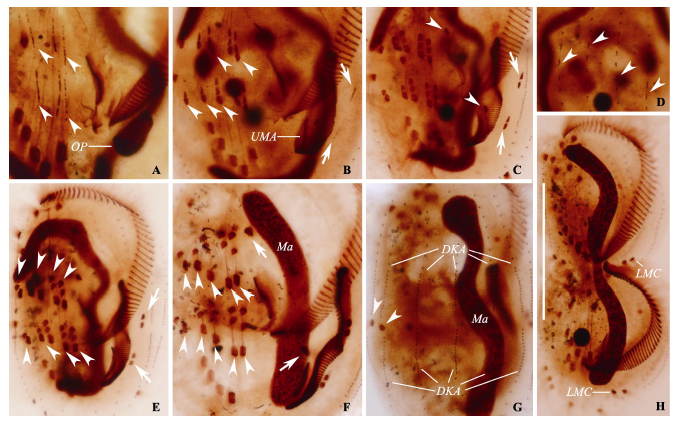
图3 小腔游仆虫细胞发生个体银染制片的显微照片。A: 发生早期个体, 示后仔虫口原基出现, 短箭头示前后仔虫的额-腹-横棘毛原基; B: 发生早中期个体腹面观, 短箭头示额-腹-横棘毛原基增粗并开始发生断裂, 长箭头示前后仔虫的缘棘毛原基; C: 发生中期个体腹面观, 示额-腹-横棘毛原基断裂形成棘毛, 短箭头示前后仔虫的最左侧一根额腹棘毛, 长箭头示缘棘毛原基开始断裂形成缘棘毛; D: 发生早中期个体背面观, 短箭头示新形成的背触毛原基; E: 发生中期个体腹面观, 短箭头示额-腹-横棘毛片段化基本完成并开始迁移, 长箭头示前后仔虫新的缘棘毛; F: 发生后期个体腹面观, 短箭头示前后仔虫的横棘毛, 长箭头示最左侧一根额腹棘毛, 此时大核呈棒状; G: 发生后期个体背面观, 短箭头示前仔虫新形成的尾棘毛; H: 发生末期个体腹面观, 示新形成棘毛基本迁移至其最终位置, 大核开始发生分裂。DKA: 背触毛原基; LMC: 左缘棘毛; Ma: 大核; OP: 后仔虫口原基; UMA: 波动膜原基。比例尺: 70 µm。
Fig. 3 Photomicrographs of Euplotes aediculatus during morphogenesis after protargol impregnation. A, Ventral view of an early divider, showing the oral primordium in opisthe, arrowheads mark the frontal-ventral-transverse cirral streaks of both dividers; B, Ventral view of an early-middle divider, arrowheads show the differentiation of frontal-ventral-transverse cirral anlagen, arrows marks the marginal anlagen; C, Ventral view of a mid-stage divider, arrowheads demonstrate the leftmost frontal cirrus (I/1) in both proter and opisthe, arrows marks the differentiation of marginal anlagen; D, Dorsal view of an early-middle divider, arrowheads show the formation of dorsal kineties anlagen; E, Ventral view of another mid-stage divider, arrowheads show the differentiation of frontal-ventral-transverse cirral anlagen almost complete, arrows point to the new marginal cirri of both daughter cells; F, Ventral view of a late divider, noting the macronucleus become a short strand, arrowheads show the transverse cirri, arrows mark the leftmost frontal cirri (I/1); G, Dorsal view of another late divider, arrowheads show the newly formed caudal cirri in the proter; H, Ventral view of a last stage divider, showing cirri almost in their final position and the division of the macronucleus. DKA, Dorsal kineties anlagen; LMC, Left marginal cirri; Ma, Macronucleus; OP, Opisthe’s oral primordium; UMA, Undulating membrane anlagen. Scale bar = 70 µm.
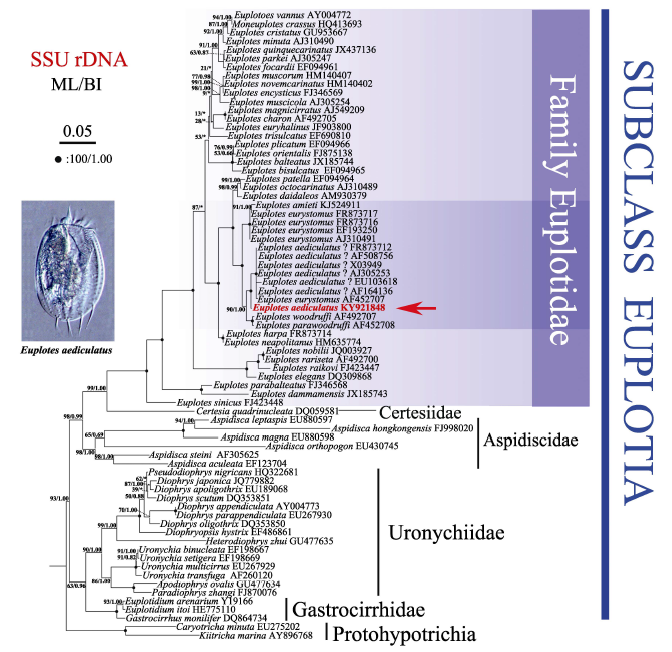
图4 基于核糖体小亚基基因序列构建的最大似然树。本文新测序列加粗显示。节点置信值以最大似然树/贝叶斯树顺序给出。黑点表示满分置信值(100/1.00), “*”表示贝叶斯树与最大似然树拓扑结构不同。比例尺代表每100个碱基位点中有5个碱基替代。
Fig. 4 Maximum likelihood (ML) tree based on small subunit ribosomal DNA sequences. Newly characterized sequence in this study is shown in bold. The numbers at the nodes represent the support values of ML/Bayesian inference (BI). Fully supported (100/1.00) branches are marked with solid circles. Asterisks reflect the disagreement between ML and BI. All branches are drawn to scale. The scale bar corresponds to five substitutions per 100 nucleotide positions.
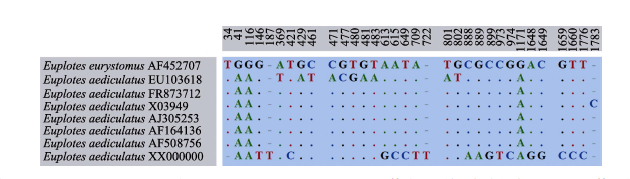
图5 7个Euplotes aediculatus种群及1个E. eurystomus种群的SSU rDNA碱基差异比较。数字代表比对后的碱基所在位置, 缺失位点用(-)表示, 比对一致的碱基用(.)表示。
Fig. 5 Unmatched sites from SSU rDNA sequence alignment of seven Euplotes aediculatus populations and one E. eurystomus population. Numbers indicate the positions of nucleotides in the alignment, missing sites are represented by dashes (-), and matching sites are marked with dots (.).
| [1] | Adl SM, Simpson AG, Lane CE, Lukes J, Bass D, Bowser SS, Brown MW, Burki F, Dunthorn M, Hampl V, Heiss A, Hoppenrath M, Lara E, Le Gall L, Lynn DH, McManus H, Mitchell EA, Mozley-Stanridge SE, Parfrey LW, Pawlowski J, Rueckert S, Shadwick L, Schoch CL, Smirnov A, Spiegel FW (2012) The revised classification of eukaryotes. Journal of Eukaryotic Microbiology, 59, 429-493. |
| [2] | Borror AC (1972) Revision of the order Hypotrichida (Ciliophora, Protozoa). Journal of Protozoology, 19, 1-23. |
| [3] | Borror AC, Hill BF (1995) The order Euplotida (Ciliophora): taxonomy, with division of Euplotes into several genera. Journal of Eukaryotic Microbiology, 42, 457-466. |
| [4] | Carter HP (1972) Infraciliature of eleven species of the genus Euplotes. Transactions of the American Microscopical Society, 91, 466-492. |
| [5] | Chen XM, Zhao Y, Al-Farraj SA, Saleh AQ, El-Serehy HA, Shao C, Al-Rasheid KAS (2015) Taxonomic descriptions of two marine ciliates, Euplotes dammamensis n. sp. and Euplotes balteatus (Dujardin, 1841) Kahl, 1932 (Ciliophora, Spirotrichea, Euplotida), collected from the Arabian Gulf, Saudi Arabia. Acta Protozoologica, 52, 73-87. |
| [6] | Corliss JO (1979) The Ciliated Protozoa:Characterization, Classification and Guide to the Literature.Pergamon Press, Oxford. |
| [7] | Curds RC (1975) A guide to the species of Euplotes (Hypotrichida, Ciliatea). Bulletin of the British Museum (Natural History) Zoology, 28, 3-61. |
| [8] | Curds RC, Wu ICH (1983) A review of the Euplotidae (Hypotrichida, Ciliophora). Bulletin of the British Museum (Natural History) Zoology, 44, 191-247. |
| [9] | Dai RH, Xu KD, He YY (2013) Morphological, physiological, and molecular evidences suggest that Euplotes parawoodruffi is a junior synonym of Euplotes woodruffi (Ciliophora, Euplotida). Journal of Eukaryotic Microbiology, 60, 70-78. |
| [10] | Di Giuseppe G, Erra F, Frontini F, Dini F, Vallesi A, Luporini P (2014) Improved description of the bipolar ciliate, Euplotes petzi, and definition of its basal position in the Euplotes phylogenetic tree. European Journal of Protistology, 50, 402-411. |
| [11] | Dong JY, Lu XT, Shao C, Huang J, Alrasheid KA (2016) Morphology, morphogenesis and molecular phylogeny of a novel saline soil ciliate, Lamtostyla salina n. sp. (Ciliophora, Hypotricha). European Journal of Protistology, 56, 219-231. |
| [12] | Dragesco J (1970) Ciliés Libres du Cameroun. Annales de la Faculté des Sciences (Numéro hors série). Université Fédérale du Cameroun, Yaoundé. (in French) |
| [13] | Dragesco J (2003) Infraciliature et morphometrie de vingt espèces de ciliés hypotriches recoltés au Rwanda et Burundi, comprenant Kahliella quadrinucleata n. sp., Pleurotricha multinucleata n. sp. et Laurentiella bergeri n. sp. Travaux du Muséum National d’Histoire Naturelle “Grigore Antipa”, 45, 7-59. (in French) |
| [14] | Foissner W (1982) Ecology and taxonomy of the Hypotrichida (Protozoa: Ciliophora) of some Austrian soils. Archiv Für Protistenkunde, 126, 19-143. (in French with English abstract) |
| [15] | Foissner W (1991) Basic light and scanning electron microscopic methods for taxonomic studies of ciliated protozoa. European Journal of Protistology, 27, 313-330. |
| [16] | Foissner W (2014) An update of ‘basic light and scanning electron microscopic methods for taxonomic studies of ciliated Protozoa’. International Journal of Systematic and Evolutionary Microbiology, 64, 271-292. |
| [17] | Fotedar R, Stoeck T, Filker S, Fell JW, Agatha S, Al Marri M, Jiang JM (2016) Description of the halophile Euplotes qatarensis nov. spec. (Ciliophora, Spirotrichea, Euplotida) isolated from the hypersaline Khor Al-Adaid Lagoon in Qatar. Journal of Eukaryotic Microbiology, 63, 578-590. |
| [18] | Gao F, Warren A, Zhang QQ, Gong J, Miao M, Sun P, Xu DP, Huang J, Yi ZZ, Song WB (2016) The all-data-based evolutionary hypothesis of ciliated protists with a revised classification of the phylum Ciliophora (Eukaryota, Alveolata). Scientific Reports, 6, 24874. |
| [19] | Hewitt EA, Müller KM, Cannone J, Hogan DJ, Gutell R, Prescott DM (2003) Phylogenetic relationships among 28 spirotrichous ciliates documented by rDNA. Molecular Phylogenetics and Evolution, 29, 258-267. |
| [20] | Jiang JM, Zhang QQ, Hu XZ, Shao C, Al-Rasheid KAS, Song WB (2010a) Two new marine ciliates, Euplotes sinicus sp. nov. and Euplotes parabalteatus sp. nov., and a new small subunit rRNA gene sequence of Euplotes rariseta (Ciliophora, Spirotrichea, Euplotida). International Journal of Systematic and Evolutionary Microbiology, 60, 1241-1251. |
| [21] | Jiang JM, Zhang QQ, Warren A, Al-Rasheid KAS, Song WB (2010b) Morphology and SSU rRNA gene-based phylogeny of two marine Euplotes species, E. orientalis spec. nov. and E. raikovi (Ciliophora, Euplotida). European Journal of Protistology, 46, 121-132. |
| [22] | Kahl A (1932) Urtiere oder Protozoa. I: Wimpertiere oder Ciliata (Infusoria), 3. Spirotricha. Tierwelt Dtl., 25, 399-650. (in German) |
| [23] | Lahr DJG, Laughinghouse HD, Oliverio A, Gao F, Katz LA (2014) How discordant morphological and molecular evolution among microorganisms can revise our notions of biodiversity on Earth. Bioessays, 36, 950-959. |
| [24] | Li LQ, Zhao XL, Ji DD, Hu XZ, Al-Rasheid KA, Al-Farraj SA, Song WB (2016) Description of two marine amphisiellid ciliates, Amphisiella milnei (Kahl, 1932) Horváth, 1950 and A. sinica sp. nov. (Ciliophora: Hypotrichia), with notes on their ontogenesis and SSU rDNA-based phylogeny. European Journal of Protistology, 54, 59-73. |
| [25] | Liu MJ, Fan YB, Miao M, Hu XZ, Al-Rasheid KAS, Al-Farraj SA, Ma HG (2015) Morphological and morphogenetic redescriptions and SSU rRNA gene-based phylogeny of the poorly-known species Euplotes amieti Dragesco, 1970 (Ciliophora, Euplotida). Acta Protozoologica, 54, 171. |
| [26] | Lynn DH (2008) The Ciliated Protozoa: Characterization, Classification and Guide to the Literature, 3rd edn. Springer-Verlag, Dordrecht. |
| [27] | Ma HG, Jiang JM, Hu XZ, Shao C, Song WB (2008) Morphology and morphogenesis of the marine ciliate, Euplotes rariseta (Ciliophora, Euplotida). Acta Hydrobiologica Sinica, 32, 57-62. |
| [28] | Medlin L, Elwood HJ, Stickel S, Sogin ML (1988) The characterization of enzymatically amplified eukaryotic 16S-like rRNA-coding regions. Gene, 71, 491-499. |
| [29] | Nylander JA (2004) MrModeltest 2. 2. Department of Systematic Zoology, Evolutionary Biology Centre, Uppsala University, Uppsala. |
| [30] | Pan HB, Hu JX, Jiang JM, Wang LQ, Hu XZ (2015) Morphology and phylogeny of three Pleuronema species (Ciliophora, Scuticociliatia) from Hangzhou Bay, China, with description of two new species, P. binucleatum n. sp. and P. parawiackowskii n. sp. Journal of Eukaryotic Microbiology, 63, 287-298. |
| [31] | Pang YB, Wei HB (1999) Studies on the morphology and morphogenesis in Euplotes aediculatus. Journal of East China Normal University (Natural Science), (1), 103-109. (in Chinese with English abstract) |
| [庞延斌, 魏红兵 (1999) 小腔游仆虫Euplotes aediculatus形态和形态发生的研究. 华东师范大学学报(自然科学版), (1), 103-109.] | |
| [32] | Petroni G, Dini F, Verni F, Rosati G (2002) A molecular approach to the tangled intrageneric relationships underlying phylogeny in Euplotes (Ciliophora, Spirotrichea). Molecular Phylogenetics and Evolution, 22, 118-130. |
| [33] | Pierson BF (1943) A comparative morphological study of several species of Euplotes closely related to Euplotes patella. Journal of Morphology, 72, 125-165. |
| [34] | Pierson BF, Gierke R, Fisher AL (1968) Clarification of the taxonomic identification of Euplotes eurystomus Kahl and E. aediculatus Pierson. Transactions of the American Microscopical Society, 87, 306-316. |
| [35] | Posada D, Crandall KA (1998) Modeltest: testing the model of DNA substitution. Bioinformatics, 14, 817-818. |
| [36] | Qu ZS, Pan HB, Hu XZ, Li JQ, Al-Farraj SA, Al-Rasheid KAS, Yi ZZ (2015) Morphology and molecular phylogeny of three cyrtophorid ciliates (Protozoa, Ciliophora) from China, including two new species, Chilodonella parauncinata sp. n. and Chlamydonella irregularis sp. n. Journal of Eukaryotic Microbiology, 62, 267-279. |
| [37] | Ronquist F, Huelsenbeck JP (2003) MRBAYES 3: Bayesian phylogenetic inference under mixed models. Bioinformatics, 19, 1572-1574. |
| [38] | Ruffolo JJ (1976) Cortical morphogenesis during the cell division cycle in Euplotes: an integrated study using light optical, scanning electron and transmission electron microscopy. Journal of Morphology, 148, 489-527. |
| [39] | Shao C, Ma HG, Gao S, Khaled ARA, Song WB (2010) Reevaluation of cortical developmental patterns in Euplotes (s. l.), including a morphogenetic redescription of E. charon (Protozoa, Ciliophora, Euplotida). Chinese Journal of Oceanology and Limnology, 28, 593-602. |
| [40] | Sogin ML, Swanton MT, Gunderson JH, Elwood HJ (1986) Sequence of the small subunit ribosomal RNA gene from the hypotrichous ciliate Euplotes aediculatus. Journal of Protozoology, 33, 26-29. |
| [41] | Song WB, Warren A, Hu XZ (2009)Free-living Ciliates in the Bohai and Yellow Seas, China. Science Press, Beijing. |
| (in Chinese and in English) [宋微波, Warren A., 胡晓钟 (2009) 中国黄渤海的自由生纤毛虫. 科学出版社, 北京.] | |
| [42] | Stamatakis A, Hoover P, Rougemont J (2008) A rapid bootstrap algorithm for the RAxML web servers. Systematic Biology, 57, 758-771. |
| [43] | Syberg-Olsen MJ, Irwin NAT, Vannini C, Erra F, Di Giuseppe G, Boscaro V, Keeling PJ (2016) Biogeography and character evolution of the ciliate genus Euplotes (Spirotrichea, Euplotia), with description of Euplotes curdsi sp. nov. PLoS ONE, 11, e0165442. |
| [44] | Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596-1599. |
| [45] | Tuffrau M (1960) Révision du genre Euplotes, fondée sur la comparaison des structures superficielles. Hydrobiologia, 15, 1-77. (in French) |
| [46] | Vannini C, Ferrantini F, Ristori A, Verni F, Petroni G (2012) Betaproteobacterial symbionts of the ciliate Euplotes: origin and tangled evolutionary path of an obligate microbial association. Environmental Microbiology, 14, 2553-2563. |
| [47] | Washburn ES, Borror AC (1972) Euplotes raikovi Agamaliev, 1966 (Ciliophora, Hypotrichida) from New Hampshire: description and morphogenesis. Journal of Protozoology, 19, 604-608. |
| [48] | Wilbert N (1975) Eine verbesserte Technik der Protargolimprä gnation für Ciliaten. Mikrokosmos, 64, 171-179. (in German) |
| [49] | Xie DM, Fan XP, Ni B, Gu FK (2016) Morphogenesis of the cortical silver-line system in the ciliate genus Euplotes (Protozoa, Ciliophora). Periodical of Ocean University of China (Natural Science), 46, 41-50. (in Chinese with English abstract) |
| [谢冬梅, 范鑫鹏, 倪兵, 顾福康 (2016) 游仆虫(原生动物, 纤毛门)皮层银线系的形态发生模式. 中国海洋大学学报(自然科学版), 46, 41-50.] | |
| [50] | Yi ZZ, Katz LA, Song WB (2012) Assessing whether alpha-tubulin sequences are suitable for phylogenetic reconstruction of Ciliophora with insights into its evolution in euplotids. PLoS ONE, 7, e40635. |
| [51] | Yi ZZ, Song WB, Clamp JC, Chen ZG, Gao S, Zhang QQ (2009) Reconsideration of systematic relationships within the order Euplotida (Protista, Ciliophora) using new sequences of the gene coding for small-subunit rRNA and testing the use of combined data sets to construct phylogenies of the Diophrys-complex. Molecular Phylogenetics and Evolution, 50, 599-607. |
| [1] | 张颂琪, 陆义, 陈炳耀, 杨光, 王彦平, 陈传武. 全球鲸豚类形态、生活史和生态学特征数据集[J]. 生物多样性, 2025, 33(2): 24442-. |
| [2] | 杨清, 张鹏, 安瑞志, 乔楠茜, 达珍, 巴桑. 拉萨河中下游纤毛虫群落时空分布模式及其驱动机制[J]. 生物多样性, 2022, 30(6): 22012-. |
| [3] | 孙翌昕, 李英滨, 李玉辉, 李冰, 杜晓芳, 李琪. 高通量测序技术在线虫多样性研究中的应用[J]. 生物多样性, 2022, 30(12): 22266-. |
| [4] | 佟一杰, 张萌娜, 万霞, 杨星科, 白明. 全球锹甲的几何形态学数据集[J]. 生物多样性, 2021, 29(9): 1159-1164. |
| [5] | 王楠, 黄菁华, 霍娜, 杨盼盼, 张欣玥, 赵世伟. 宁南山区不同植被恢复方式下土壤线虫群落特征:形态学鉴定与高通量测序法比较[J]. 生物多样性, 2021, 29(11): 1513-1529. |
| [6] | 王伟, 刘阳. 植物生命之树重建的现状、问题和对策建议[J]. 生物多样性, 2020, 28(2): 176-188. |
| [7] | 陈作艺, 许晓静, 朱素英, 翟梦怡, 李扬. 中国沿海洛氏角毛藻复合群的多样性组成及地理分布[J]. 生物多样性, 2019, 27(2): 149-158. |
| [8] | 王伟, 张晓霞, 陈之端, 路安民. 被子植物APG分类系统评论[J]. 生物多样性, 2017, 25(4): 418-426. |
| [9] | 孙航, 邓涛, 陈永生, 周卓. 植物区系地理研究现状及发展趋势[J]. 生物多样性, 2017, 25(2): 111-122. |
| [10] | 谢平. 浅析物种概念的演变历史[J]. 生物多样性, 2016, 24(9): 1014-1019. |
| [11] | 张静, 李渊, 宋娜, 林龙山, 高天翔. 我国沿海棱鳀属鱼类的物种鉴定与系统发育[J]. 生物多样性, 2016, 24(8): 888-895. |
| [12] | 王超锋, 李海波, 张武昌, 赵丽, 赵苑, 肖天. 2013年夏威夷东南部海区表层砂壳纤毛虫的群落结构和分布特征[J]. 生物多样性, 2016, 24(7): 831-837. |
| [13] | 陈雪, 张武昌, 吴强, 栾青杉, 肖天. 莱州湾大型砂壳纤毛虫群落季节变化[J]. 生物多样性, 2014, 22(5): 649-657. |
| [14] | 杜周和, 刘俊凤, 刘彬斌, 左艳春, 吴建梅, 陈义安, 张剑飞, 鲁成. 家蚕地方品种遗传多样性及其分子系统学研究[J]. 生物多样性, 2013, 21(3): 315-325. |
| [15] | 黄继红, 张金龙, 杨永, 马克平. 特有植物多样性分布格局测度方法的新进展[J]. 生物多样性, 2013, 21(1): 99-110. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
![]()