



生物多样性 ›› 2019, Vol. 27 ›› Issue (11): 1205-1220. DOI: 10.17520/biods.2019316 cstr: 32101.14.biods.2019316
收稿日期:2019-10-09
接受日期:2019-12-17
出版日期:2019-11-20
发布日期:2020-01-17
通讯作者:
卫然
基金资助:
Siqi Liang1,2,Xianchun Zhang1,Ran Wei1,*( )
)
Received:2019-10-09
Accepted:2019-12-17
Online:2019-11-20
Published:2020-01-17
Contact:
Wei Ran
摘要:
广泛的杂交和多倍化使得铁角蕨属(Asplenium)下存在着许多分类困难的物种复合体, 针对这些类群进行整合分类学的研究, 有助于我们更加全面和深入地理解物种的界限以及形成机制。线裂铁角蕨复合体(Asplenium coenobiale complex)是铁角蕨属下一个形态多样性较高的类群, 由于缺乏全面取样和系统研究, 该复合体的物种划分长期存在争议。本研究选取线裂铁角蕨复合体中形态变异和地理分布具有代表性的个体, 通过孢粉学研究确定该类群的生殖特性, 运用流式细胞分析获取倍性信息, 同时结合叶绿体和核基因组片段系统发生分析的证据, 对该类群的系统演化关系和起源方式进行了探讨。结果表明: (1)虽然部分孢子囊败育的情况在线裂铁角蕨复合体中十分普遍, 但正常孢子囊内形成的64个孢子说明该类群植物仍能进行正常的有性生殖; (2)该复合体中存在着倍性变异, 其中多角铁角蕨(A. cornutissimum)是二倍体, 而其他成员均为四倍体; (3)依据母系遗传的叶绿体序列所构建的系统发生关系将该类群划为4个分支, 与基于核基因序列构建的系统树存在冲突, 这暗示杂交可能在该复合体的形成过程中起到了重要的推动作用。综上所述, 我们建议将线裂铁角蕨复合体划分为4个物种, 即同源四倍体新种马关铁角蕨(A. maguanense sp. nov.), 二倍体多角铁角蕨, 以及两个由同一对亲本正反交产生的异源四倍体线裂铁角蕨(A. coenobiale)和叶基宽铁角蕨(A. pulcherrimum)。
梁思琪, 张宪春, 卫然 (2019) 利用整合分类学方法进行蕨类植物复合体的物种划分: 以线裂铁角蕨复合体为例. 生物多样性, 27, 1205-1220. DOI: 10.17520/biods.2019316.
Siqi Liang, Xianchun Zhang, Ran Wei (2019) Integrative taxonomy resolved species delimitation in a fern complex: A case study of the Asplenium coenobiale complex. Biodiversity Science, 27, 1205-1220. DOI: 10.17520/biods.2019316.

图1 线裂铁角蕨复合体的叶片分裂程度变异(比例尺均为2 cm)。 (A)叶基宽铁角蕨(8573)全株, 二回羽状至羽状全裂; (B)叶基宽铁角蕨(9525)叶片, 二回羽状至三回羽状-羽状全裂; (C)线裂铁角蕨(9439-1)全株, 二回羽状至羽状半裂; (D)线裂铁角蕨(9524-6)叶, 二回羽状至羽状全裂; (E)线裂铁角蕨(9524-10)全株, 二回羽状至三回羽状-羽状半裂; (F)叶基宽铁角蕨(9443)基部羽片, 三回羽状至四回羽状-羽状全裂; (G)叶基宽铁角蕨(9524-1)基部羽片, 三回羽状至四回羽状-羽状全裂; (H)线裂铁角蕨(9524-17)叶片, 二回羽状至三回羽状-羽状半裂; (I)线裂铁角蕨(9524-4)基部羽片, 三回羽状至羽状全裂; (J)线裂铁角蕨(9524-16)叶片, 二回羽状。
Fig. 1 Variation of the frond division within the Asplenium coenobiale complex (Scale bar = 2 cm). (A) Habit of A. pulcherrimum (8573), bipinnate-pinnatisect; (B) Lamina of A. pulcherrimum (9525), bipinnate to tripinnate-pinnatisect; (C) Habit of A. coenobiale (9439-1), bipinnate-pinnatifid; (D) Frond of A. coenobiale (9524-6), bipinnate-pinnatisect; (E) Habit of A. coenobiale (9524-10), bipinnate to tripinnate-pinnatifid; (F) Basal pinnae A. pulcherrimum (9443), tripinnate to quadripinnatisect; (G) Basal pinnae A. pulcherrimum (9524-4), tripinnate to quadripinnatisect; (H) Lamina of A. coenobiale (9524-17), bipinnate to tripinnate-pinnatifid; (I) Basal pinnae A. coenobiale (9443), tripinnate-pinnatisect; (J) Lamina of A. coenobiale (9524-16), bipinnate.
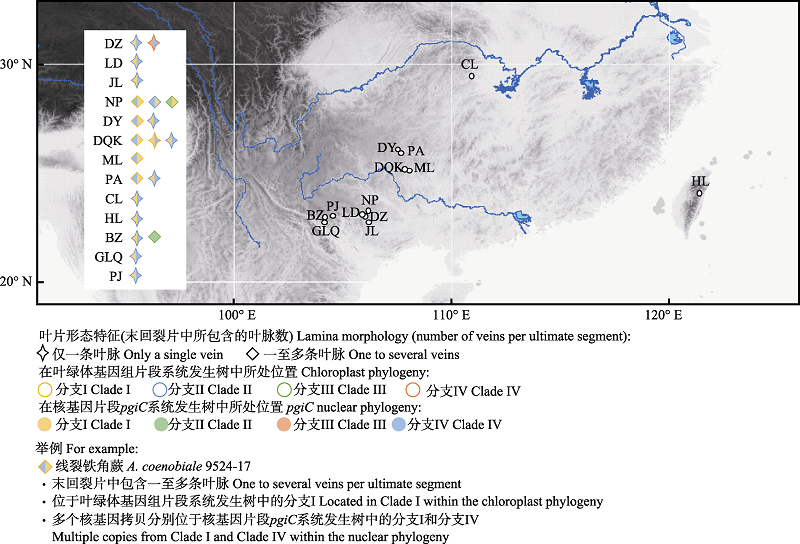
图2 线裂铁角蕨复合体的采样分布图。DZ、LD、JL、NP、DY、DQK、ML、PA、CL、HL、BZ、GLQ、PJ含义同表1。
Fig. 2 Map of localities of the examined accessions of the Aspelnium coenobiale complex. DZ, LD, JL, NP, DY, DQK, ML, PA, CL, HL, BZ, GLQ, PJ are the same as Table 1.
| 片段 Fragment | 引物 Primer | 引物序列(5′-3′) Primer sequence (5′-3′) | 最佳碱基替代模型 Best substitution model | 长度 Length (bp) | 参考文献 Reference |
|---|---|---|---|---|---|
| trnL-trnF | Fern-1 | GGCAGCCCCCARATTCAGGGRAACC | K81UF+G | 693 | Trewick et al, 2002 |
| f | ATTTGAACTGGTGACACGAG | Taberlet et al, 1991 | |||
| rbcL | 1F | ATGTCACCACAAACAGA(G/A)ACTAAAGC | GTR+I+G | 1,197 | Gastony & Rollo, 1995 |
| 1351R | CTTCACAAGCAGCAGCTAGTTCAGGACTCC | Gastony & Rollo, 1995 | |||
| rpl32-trnP | 112F | TCCATCTTAACCGGTCGTCGTTCA | TVM+G | 644 | Liang et al, 2019 |
| 858R | AGTTTGGTAGCGCGTCATCT | Liang et al, 2019 | |||
| pgiC | 14F | GTGCTTCTGGGTCTTTTGAGTG | HKY+F+G4 | 648 | Ishikawa et al, 2002 |
| 16R | GTTGTCCATTAGTTCCAGGTTCCCC | Ishikawa et al, 2002 |
表2 本研究所采用的分子标记信息
Table 2 Information of molecular markers in this study
| 片段 Fragment | 引物 Primer | 引物序列(5′-3′) Primer sequence (5′-3′) | 最佳碱基替代模型 Best substitution model | 长度 Length (bp) | 参考文献 Reference |
|---|---|---|---|---|---|
| trnL-trnF | Fern-1 | GGCAGCCCCCARATTCAGGGRAACC | K81UF+G | 693 | Trewick et al, 2002 |
| f | ATTTGAACTGGTGACACGAG | Taberlet et al, 1991 | |||
| rbcL | 1F | ATGTCACCACAAACAGA(G/A)ACTAAAGC | GTR+I+G | 1,197 | Gastony & Rollo, 1995 |
| 1351R | CTTCACAAGCAGCAGCTAGTTCAGGACTCC | Gastony & Rollo, 1995 | |||
| rpl32-trnP | 112F | TCCATCTTAACCGGTCGTCGTTCA | TVM+G | 644 | Liang et al, 2019 |
| 858R | AGTTTGGTAGCGCGTCATCT | Liang et al, 2019 | |||
| pgiC | 14F | GTGCTTCTGGGTCTTTTGAGTG | HKY+F+G4 | 648 | Ishikawa et al, 2002 |
| 16R | GTTGTCCATTAGTTCCAGGTTCCCC | Ishikawa et al, 2002 |
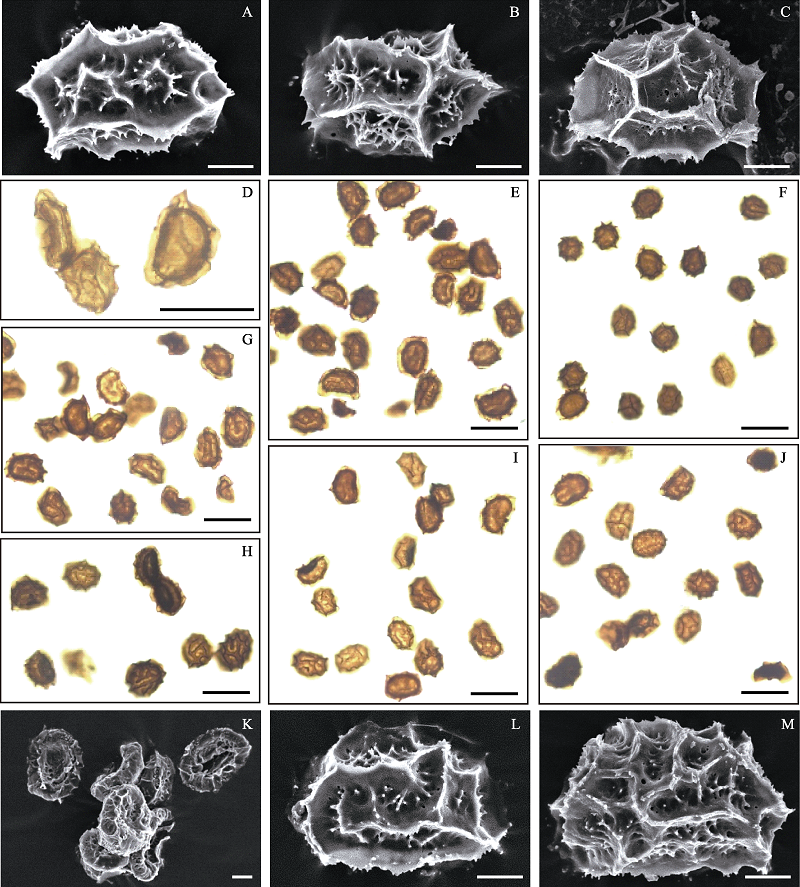
图3 线裂铁角蕨复合体的孢子。 (A) (似)线裂铁角蕨(8214), 正常孢子, 孢子外壁具脊(鸡冠状-翅状); (B)(似)线裂铁角蕨(9439-2), 正常孢子, 孢子外壁具脊(鸡冠状-翅状); (C)多角铁角蕨(9444), 正常孢子, 孢子外壁具翅; (D) (似)线裂铁角蕨(8214), 正常孢子(肾形, 饱满)和败育孢子(干瘪); (E) (似)线裂铁角蕨(9439-2), 正常孢子和畸形且发黑的孢子; (F)多角铁角蕨(9444), 正常孢子, 明显小于该复合体中其他类群的孢子; (G)线裂铁角蕨(9524-10), 正常孢子和干瘪、畸形的孢子; (H)线裂铁角蕨(9524-16), 正常孢子和畸形孢子; (I)叶基宽铁角蕨(9524-1), 正常孢子和发黑的孢子; (J)叶基宽铁角蕨(9443), 正常孢子和畸形、发黑的孢子; (K)线裂铁角蕨(8057), 干瘪的败育孢子; (L)线裂铁角蕨(9524-17), 正常孢子, 孢子外壁具脊(鸡冠状-翅状); (M)叶基宽铁角蕨(8059B), 正常孢子, 孢子外壁具脊(鸡冠状-翅状)。A-C, K-M中比例尺为10 μm; D-J中比例尺为50 μm。
Fig. 3 Spores of the Asplenium coenobiale complex. (A) A. aff. coenobiale (8214), a normal spore, perispore lophate (cristate-alate); (B) A. aff. coenobiale (9439-2), a normal spore, perispore lophate (cristate-alate); (C) A. cornutissimum (9444), a normal spore, perispore alate; (D) A. aff. coenobiale (8214), a normal spore (kidney-shaped, well-filled) and abortive spores (shrivelled); (E) A. aff. coenobiale (9439-2), normal spores and several misshapen, blackened spores; (F) A. cornutissimum (9444), normal spores, and the size of which is relatively small compared with other accessions’ spores; (G) A. coenobiale (9524-10), normal spores and several misshapen, shrivelled spores; (H) A. coenobiale (9524-16), normal spores and several misshapen spores; (I) A. pulcherrimum (9524-1), normal spores and several blackened spores; (J) A. pulcherrimum (9443), normal spores and several misshapen, blackened spores; (K) A. coenobiale (8057), shrivelled, abortive spores; (L) A. coenobiale (9524-17), a normal spore, perispore lophate (cristate-alate); (M) A. pulcherrimum (8059B), a normal spore, perispore lophate (cristate–alate). Scale bars in A–Cand K–M are 10 μm; scale bars in D–J are 50 μm.
| 凭证标本 Voucher specimen | 核DNA含量 Nuclear DNA content (pg) | 孢子外壁长度 Length of exospore (μm) | 推定倍性 Inferred ploidy level | 孢子囊内孢子数(统计的孢子囊个数) Spore number per sporangium (Number of checked sporangia) | |
|---|---|---|---|---|---|
| 2C | 1Cx | ||||
| A. cornutissimum 9444 | 11.3 ± 0.5 | 5.7 ± 0.2 | (22-) 25-27-29 (-30) | 2x | 64 (10) |
| A. pulcherrimum 9443 | 15.8 ± 0.1 | 4.0 ± 0.0 | (29-) 32-35-37 (-39) | 4x | 64 (4) |
| A. pulcherrimum 9525 | 16.1 ± 0.5 | 4.0 ± 0.1 | (26-) 30-33-36 (-37) | 4x | 64 (2) |
| A. pulcherrimum 8059B | 16.7 | 4.2 | - | 4x | 64 (2) |
| A. pulcherrimum 9524-1 | 17.3 ± 0.2 | 4.3 ± 0.0 | (28-) 31-34-38 (-41) | 4x | 64 (6) |
| A. coenobiale 9524-13 | 17.4 ± 0.0 | 4.3 ± 0.0 | - | 4x | 64 (6) |
| A. pulcherrimum 9440 | 17.5 ± 0.2 | 4.4 ± 0.1 | - | 4x | - |
| A. coenobiale 9524-10 | 17.6 ± 0.4 | 4.4 ± 0.1 | (28-) 29-33-36 (-41) | 4x | 64 (4) |
| A. coenobiale 8059A | 17.9 | 4.5 | - | 4x | - |
| A. coenobiale 8057 | 18.3 ± 0.1 | 4.6 ± 0.0 | - | 4x | 64 (4) |
| A. coenobiale 9505 | 18.3 ± 0.1 | 4.6 ± 0.0 | - | 4x | 64 (3) |
| A. coenobiale 9524-17 | 18.3 ± 0.2 | 4.6 ± 0.0 | - | 4x | 64 (2) |
| A. coenobiale 9524-16 | 18.3 ± 0.3 | 4.6 ± 0.1 | (30-) 31-33-35 (-37) | 4x | 64 (4) |
| A. coenobiale 9439-1 | 18.7 ± 0.2 | 4.7 ± 0.1 | - | 4x | 64 (2) |
| A. coenobiale 9524-4 | 18.7 ± 0.4 | 4.7 ± 0.1 | - | 4x | 64 (4) |
| A. aff. coenobiale 9439-2 | 21.2 ± 0.2 | 5.3 ± 0.1 | (28-) 30-33-35 (-39) | 4x | 64 (1) |
| A. aff. coenobiale 8214 | 21.2 ± 0.2 | 5.3 ± 0.1 | (29-) 31-33-35 (-36) | 4x | 64 (8) |
| A. pulcherrimum 8245 | - | - | (27-) 30-32-35 (-39) | 4x | 64 (3) |
| A. pulcherrimum 2016002 | - | - | - | - | 64 (4) |
| A. coenobiale 2016017 | - | - | - | - | 64 (2) |
表3 线裂铁角蕨复合体细胞学和孢粉学研究结果。-: 数据缺失。
Table 3 Results from cytological and palynological examinations of the Asplenium coenobiale complex. -, Data missing.
| 凭证标本 Voucher specimen | 核DNA含量 Nuclear DNA content (pg) | 孢子外壁长度 Length of exospore (μm) | 推定倍性 Inferred ploidy level | 孢子囊内孢子数(统计的孢子囊个数) Spore number per sporangium (Number of checked sporangia) | |
|---|---|---|---|---|---|
| 2C | 1Cx | ||||
| A. cornutissimum 9444 | 11.3 ± 0.5 | 5.7 ± 0.2 | (22-) 25-27-29 (-30) | 2x | 64 (10) |
| A. pulcherrimum 9443 | 15.8 ± 0.1 | 4.0 ± 0.0 | (29-) 32-35-37 (-39) | 4x | 64 (4) |
| A. pulcherrimum 9525 | 16.1 ± 0.5 | 4.0 ± 0.1 | (26-) 30-33-36 (-37) | 4x | 64 (2) |
| A. pulcherrimum 8059B | 16.7 | 4.2 | - | 4x | 64 (2) |
| A. pulcherrimum 9524-1 | 17.3 ± 0.2 | 4.3 ± 0.0 | (28-) 31-34-38 (-41) | 4x | 64 (6) |
| A. coenobiale 9524-13 | 17.4 ± 0.0 | 4.3 ± 0.0 | - | 4x | 64 (6) |
| A. pulcherrimum 9440 | 17.5 ± 0.2 | 4.4 ± 0.1 | - | 4x | - |
| A. coenobiale 9524-10 | 17.6 ± 0.4 | 4.4 ± 0.1 | (28-) 29-33-36 (-41) | 4x | 64 (4) |
| A. coenobiale 8059A | 17.9 | 4.5 | - | 4x | - |
| A. coenobiale 8057 | 18.3 ± 0.1 | 4.6 ± 0.0 | - | 4x | 64 (4) |
| A. coenobiale 9505 | 18.3 ± 0.1 | 4.6 ± 0.0 | - | 4x | 64 (3) |
| A. coenobiale 9524-17 | 18.3 ± 0.2 | 4.6 ± 0.0 | - | 4x | 64 (2) |
| A. coenobiale 9524-16 | 18.3 ± 0.3 | 4.6 ± 0.1 | (30-) 31-33-35 (-37) | 4x | 64 (4) |
| A. coenobiale 9439-1 | 18.7 ± 0.2 | 4.7 ± 0.1 | - | 4x | 64 (2) |
| A. coenobiale 9524-4 | 18.7 ± 0.4 | 4.7 ± 0.1 | - | 4x | 64 (4) |
| A. aff. coenobiale 9439-2 | 21.2 ± 0.2 | 5.3 ± 0.1 | (28-) 30-33-35 (-39) | 4x | 64 (1) |
| A. aff. coenobiale 8214 | 21.2 ± 0.2 | 5.3 ± 0.1 | (29-) 31-33-35 (-36) | 4x | 64 (8) |
| A. pulcherrimum 8245 | - | - | (27-) 30-32-35 (-39) | 4x | 64 (3) |
| A. pulcherrimum 2016002 | - | - | - | - | 64 (4) |
| A. coenobiale 2016017 | - | - | - | - | 64 (2) |
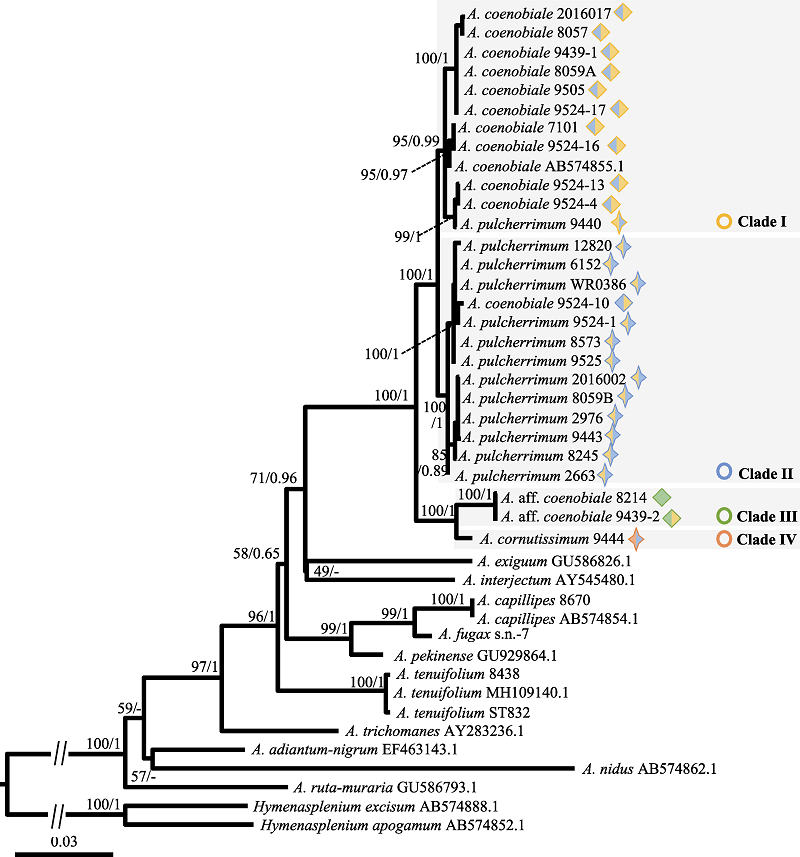
图4 基于叶绿体基因组3个DNA片段进行最大似然分析获得的系统发生树。系统发生分析的支持率表示顺序为: 最大似然法的自展支持率(BSML)和贝叶斯分析的后验概率(PPBI)。图形含义同图2。
Fig. 4 The phylogenetic tree inferred from the maximum likelihood (ML) analysis based on three chloroplast DNA sequences. Numbers above the branches indicate bootstrap values or posterior probability by ML (BSML)/Bayesian inference (PPBI). Meanings of symbols are the same as Fig. 2.
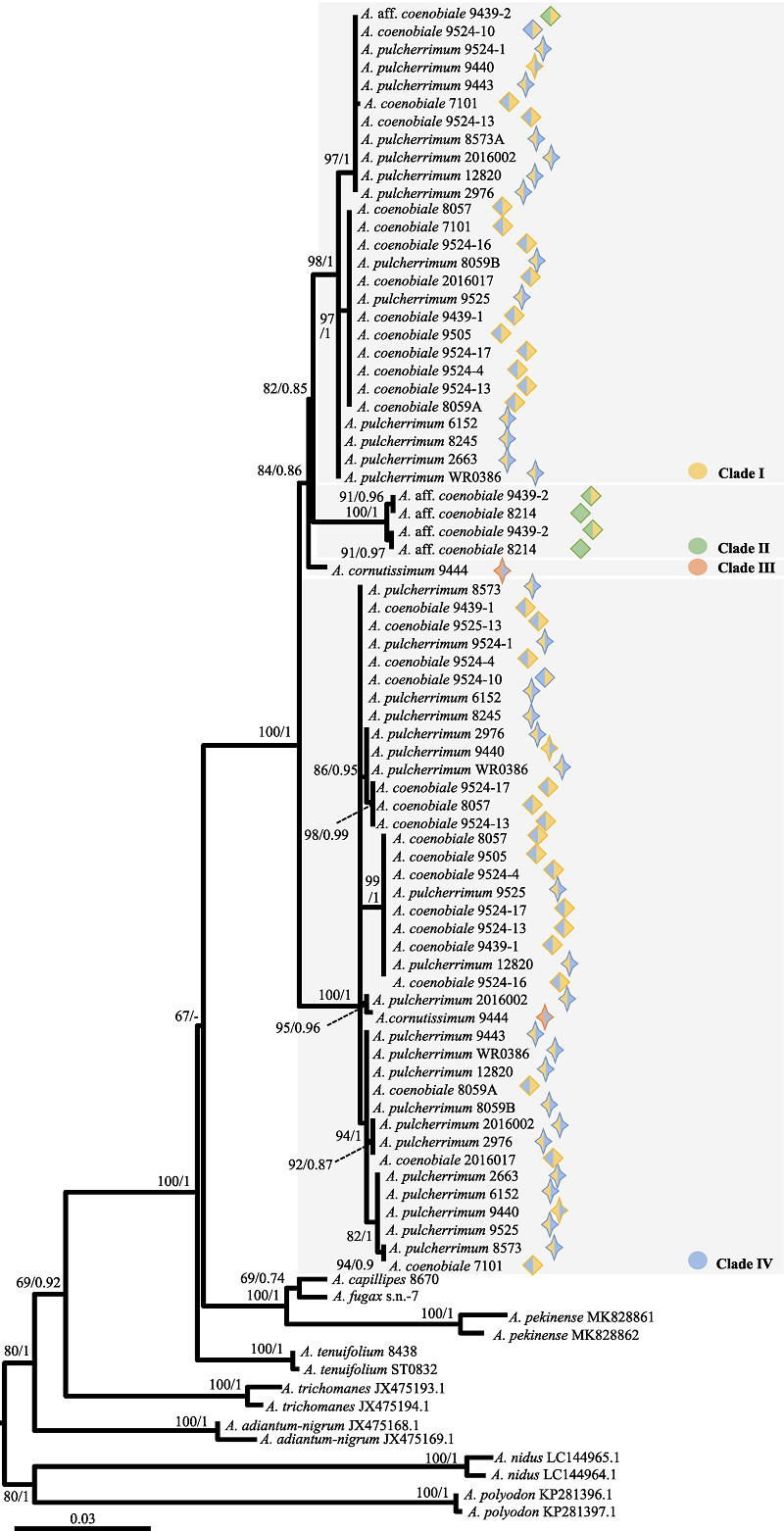
图5 基于核基因pgiC序列进行最大似然分析获得的系统发生树。系统发生分析的支持率表示顺序为: 最大似然法的自展支持率(BSML)和贝叶斯分析的后验概率(PPBI)。图形含义同图2。
Fig. 5 The phylogenetic tree inferred from the maximum likelihood analysis based on pgiC sequence. Numbers above the branches indicate bootstrap values or posterior probability by ML (BSML)/Bayesian inference (PPBI). Meanings of symbols are the same as Fig. 2.

图6 马关铁角蕨(8214)。(A)全株; (B)叶片远轴面; (C)叶片近轴面; (D)叶片基部羽片近轴面; (E)叶片中部羽片远轴面; (F)孢子囊群; (G)末回裂片顶端, 示齿和排水器; (H)鳞片。疑似杂交种(9439-2): (I)生境; (J)全株; (K)末回裂片远轴面。A-C, J-K中比例尺为2 cm; D-H中比例尺为1 mm。
Fig. 6 A. maguanense sp. nov. (8214). (A) Habit; (B) Abaxial side of lamina; (C) Adaxial side of lamina; (D) Adaxial side of basal pinna; (E) Abaxial side of middle pinna; (F) Sorus; (G) Abaxial side of ultimate segment, apex with teeth and hydathodes; (H) Scales. A. aff. coenobiale (9439-2), a suspected hybrid shared half genome with A. maguanense: (I) Habitat; (J) Habit; (K) Abaxial side of ultimate segment. Scale bars in A-C and J-K are 2 cm; scale bars in D-H are 1 mm.
| 1 | Akaike H ( 1973) Information theory as an extension of the maximum likelihood principle. In: Second International Symposium on Information Theory (eds Petrov BN, Csaki F), pp. 267-281. Akademiai Kiado, Budapest. |
| 2 | Barrington DS, Paris CA, Ranker TA ( 1986) Systematic inferences from spore and stomate size in the ferns. American Fern Journal, 76, 149-159. |
| 3 | Chang YF ( 2017) Polyploidy and the formation of species diversity in Aspleniaceae. Biodiversity Science, 25, 621-626. (in Chinese with English abstract) |
| [ 常艳芬 ( 2017) 铁角蕨科的多倍化与物种多样性形成. 生物多样性, 25, 612-626.] | |
| 4 | Chang YF, Ebihara A, Lu SG, Liu HM, Schneider H ( 2018) Integrated taxonomy of the Asplenium normale complex (Aspleniaceae) in China and adjacent areas. Journal of Plant Research, 131, 573-587. |
| 5 | DeVol CE ( 1975) Aspleniaceae. In: Flora of Taiwan, Vol. 1 (ed. Editorial Committee of Flora of Taiwan). Epoch Publishing, Taipei. |
| 6 | Díez CM, Gaut BS, Meca E, Scheinvar E, Montes-Hernandez S, Eguiarte LE, Tenaillon MI ( 2013) Genome size variation in wild and cultivated maize along altitudinal gradients. New Phytologist, 199, 264-276. |
| 7 | Dyer RJ, Savolainen V, Schneider H ( 2012) Apomixis and reticulate evolution in the Asplenium monanthes fern complex. Annals of Botany, 110, 1515-1529. |
| 8 | Ebihara A, Nitta JH, Ito M ( 2010) Molecular species identification with rich floristic sampling: DNA barcoding the pteridophyte flora of Japan. PLoS ONE, 5, e15136. |
| 9 | Gastony GJ, Rollo DR ( 1995) Phylogeny and generic circumscription of cheilanthoid ferns (Pteridaceae: Cheilanthoideae) inferred from rbcL nucleotide sequences. American Fern Journal, 85, 341-360. |
| 10 | Hall TA ( 1999) BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/ NT. Nucleic Acids Symposium Series, 41, 95-98. |
| 11 | Ishikawa H, Watano Y, Kano K, Ito M, Kurita S ( 2002) Development of primer sets for PCR amplification of the pgiC gene in ferns. Journal of Plant Research, 115, 65-70. |
| 12 | Jiang RH, Zhang XC, Liu Y ( 2011) Asplenium cornutissimum (Aspleniaceae), a new species from karst caves in Guangxi, China. Brittonia, 63, 83-86. |
| 13 | Kalyaanamoorthy S, Minh BQ, Wong TKF, von Haeseler A, Jermiin LS ( 2017) ModelFinder: Fast model selection for accurate phylogenetic estimates. Nature Methods, 14, 587-589. |
| 14 | Kumar S, Stecher G, Tamura K ( 2016) MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Molecular Biology and Evolution, 33, 1870-1874. |
| 15 | Lanfear R, Frandsen PB, Wright AM, Senfeld T, Calcott B ( 2016) PartitionFinder 2: New methods for selecting partitioned models of evolution for molecular and morphological phylogenetic analyses. Molecular Biology and Evolution, 34, 772-773. |
| 16 | Leitch IJ, Bennett MD ( 2004) Genome downsizing in polyploid plants. Biological Journal of the Linnean Society, 82, 651-663. |
| 17 | Li JZ ( 2004) Aspleniaceae. In: Flora of Hunan, Vol. 1 (eds Li JZ,Chen SM, Lin QZ).Hunan Science & Technology Press, Changsha. (in Chinese) |
| [ 李建宗 ( 2004) 铁角蕨科. 见: 湖南植物志第一卷 (李建宗, 陈三茂, 林亲众编). 湖南科学技术出版社, 长沙.] | |
| 18 | Liang SQ, Viane RLL, Zhang XC, Wei R ( 2019) Exploring the reticulate evolution in the Asplenium pekinense complex and the A. varians complex (Aspleniaceae). Journal of Systematics and Evolution, doi: 10.1111/jse.12530. |
| 19 | Lin YX, Sleep A ( 1989) A cytogenetic study of two Asplenium species from Eastern Asia: A. sarelii and A. pekinense (Aspleniaceae: Pteridophyta). In: Proceedings of the International Symposium on Systematic Pteridology (eds Shing KH, Kramer KU), pp. 111-127. China Science and Technology Press, Beijing. |
| 20 | Lin YX, Viane R ( 2013) Aspleniaceae. In: Flora of China, Vol.2-3(eds Wu ZY, Raven P, Hong DY). Science Press, Beijing & Missouri Botanical Garden Press, St. Louis. |
| 21 | Lovis JD ( 1978) Evolutionary patterns and processes in ferns. Advances in Botanical Research, 4, 229-415. |
| 22 | Lovis JD, Reichstein T ( 1964) A diploid form of Asplenium ruta-muraria. British Fern Gazette, 9, 141-146. |
| 23 | Mallet J ( 2007) Hybrid speciation. Nature, 446, 279-283. |
| 24 | Nguyen LT, Schmidt HA, von Haeseler A, Minh BQ ( 2015) IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Molecular Biology and Evolution, 32, 268-274. |
| 25 | Ohlsen DJ, Perrie LR, Shepherd LD, BrownseyPJ, Bayly MJ ( 2015) Investigation of species boundaries and relationships in the Asplenium paleaceum complex (Aspleniaceae) using AFLP fingerprinting and chloroplast and nuclear DNA sequences. Australian Systematic Botany, 27, 378-394. |
| 26 | Otto F ( 1990) DAPI staining of fixed cells for high-resolution flow cytometry of nuclear DNA. In: Methods in Cell Biology (eds Crissman HA, Dsrzynkiewicz Z), pp. 105-110. Academic Press, New York. |
| 27 | PPG I ( 2016) A community based classification of ferns and lycophytes. Journal of Systematics and Evolution, 54, 563-603. |
| 28 | Rieseberg LH, Willis JH ( 2007) Plant speciation. Science, 317, 910-914. |
| 29 | Ronquist F, Teslenko M, van der Mark P, Ayres DL, Darling A, Höhna S, Larget B, Liu L, Suchard MA, Huelsenbeck JP ( 2012) MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Systematic Biology, 61, 539-542. |
| 30 | Schneider H, Liu HM, Chang YF, Ohlsen D, Perrie LR, Shepherd L, Kessler M, Karger D, Hennequin S, Marquardt J, Russell S, Ansell S, Lu NT, Kamau P, Regalado JL, Heinrichs L, Ebihara J, Smith A, Gibby M ( 2017) Neo- and Palaeopolyploidy contribute to the species diversity of Asplenium—The most species rich genus of ferns. Journal of Systematics and Evolution, 55, 353-364. |
| 31 | Shang H, Yan YH ( 2017) Natural hybridization and biodiversity conservation. Biodiversity Science, 25, 683-688. (in Chinese with English abstract) |
| [ 商辉, 严岳鸿 ( 2017) 自然杂交与生物多样性保护. 生物多样性, 25, 683-688.] | |
| 32 | Sigel EM ( 2016) Genetic and genomic aspects of hybridization in ferns. Journal of Systematics and Evolution, 54, 638-655. |
| 33 | Soltis DE, Visger CJ, Soltis PS ( 2014) The polyploidy revolution then…and now: Stebbins revisited. American Journal of Botany, 101, 1057-1078. |
| 34 | Soltis PS, Marchant DB, van de Peer Y, Soltis DE ( 2015) Polyploidy and genome evolution in plants. Current Opinion in Genetics & Development, 35, 119-125. |
| 35 | Soltis PS, Soltis DE ( 2009) The role of hybridization in plant speciation. Annual Review of Plant Biology, 60, 561-588. |
| 36 | Taberlet P, Gielly L, Pautou G, Bouvet J ( 1991) Universal primers for amplification of three non-coding regions of chloroplast DNA. Plant Molecular Biology, 17, 1105-1109. |
| 37 | Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG ( 1997) The CLUSTAL_X Windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876-4882. |
| 38 | Trewick SA, Morgan-Richards M, Russell SJ, Henderson S, Rumsey FJ, Pinter I, Barrett JA, Gibby M, Vogel JC ( 2002) Polyploidy, phylogeography and Pleistocene refugia of the rockfern Asplenium ceterach: Evidence from chloroplast DNA. Molecular Ecology, 11, 2003-2012. |
| 39 | Viane RLL, Reichstein T ( 2003) Notes on new or interesting Asplenium species from western Asia, including comments on Ching & Wu (1985), and Fraser-Jenkins (1992) Reliquiae Reichsteinianae 1. In: Pteridology in the New Millennium (eds Chandra S, Srivastava M), pp. 73-105. Springer, Dordrecht. |
| 40 | Vogel JC, Russell SJ, Rumsey FJ, Barrett JA, Gibby M ( 1998) Evidence for maternal transmission of chloroplast DNA in the genus Asplenium (Aspleniaceae, Pteridophyta). Botanica Acta, 111, 247-249. |
| 41 | Wagner WH ( 1954) Reticulate evolution in the Appalachian Asplenium. Evolution, 8, 103-118. |
| 42 | Wang PS, Wang XY ( 2001) Aspleniaceae. In: Pteridophyte Flora of Guizhou (eds Wang PS, Wang XY). Guizhou Science & Technology Publishing House, Guiyang. (in Chinese) |
| [ 王培善, 王筱英 ( 2001) 铁角蕨科. 见:贵州蕨类植物志(王培善, 王筱英编). 贵州科技出版社, 贵阳.] | |
| 43 | Wang YG ( 2017) Natural hybridization and speciation. Biodiversity Science, 25, 565-576. (in Chinese with English abstract) |
| [ 王玉国 ( 2017) 自然杂交与物种形成. 生物多样性, 25, 565-576.] | |
| 44 | Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH ( 2009) The frequency of polyploid speciation in vascular plants. Proceedings of the National Academy of Sciences, USA, 106, 13875-13879. |
| 45 | Wu ZH ( 1999) Aspleniaceae. In: Flora Reipublicae Popularis Sinicae (ed. Editorial Committee of Flora Reipublicae Popularis Sinicae, Chinese Academy of Sciences), Tomus 4(2).Science Press, Beijing. (in Chinese) |
| [ 吴兆洪 ( 1999) 铁角蕨科. 见:中国植物志, 第4卷, 第2分册 (中国科学院中国植物志编辑委员会编). 科学出版社, 北京.] | |
| 46 | Wu ZH ( 2006) Aspleniaceae. In: Flora of Guangdong, Vol. VII (ed. Wu TL). Guangdong Science and Technology Press, Guangzhou. (in Chinese) |
| [ 吴兆洪 ( 2006) 铁角蕨科. 见:广东植物志, 第7卷 (吴德邻编).广东科技出版社, 广州.] | |
| 47 | Xu KW, Zhang L, Rothfels CJ, Smith AR, Viane R, Lorence D, Wood KR, Chen CW, Knapp R, Zhou L, Lu NT, Zhou XM, Wei HJ, Fan Q, Chen SF, Cicuzza D, Gao XF, Liao WB, Zhang LB ( 2019) A global plastid phylogeny of the fern genus Asplenium (Aspleniaceae). Cladistics , doi: 10.1111/cla. |
| 12384. | |
| 48 | Yakimowski SB, Rieseberg LH ( 2014) The role of homoploid hybridization in evolution: A century of studies synthesizing genetics and ecology. American Journal of Botany, 101, 1247-1258. |
| 49 | Zhang D, Gao FL, Li WX, Jakovlić I, Zou H, Zhang J, Wang GT ( 2018) PhyloSuite: An integrated and scalable desktop platform for streamlined molecular sequence data management and evolutionary phylogenetics studies. bioRxiv , doi: 10.1101/489088. |
| 50 | Zhang GF ( 2006) Aspleniaceae. In: Flora Yunnanica, Tomus 20 (Pterisophyta) (ed. Zhu WM). Science Press, Beijing. (in Chinese) |
| [ 张光飞 (2006) 铁角蕨科. 见:云南植物志, 第二十卷 (蕨类植物) (朱维明编). 科学出版社, 北京.] |
| [1] | 蒲佳佳, 杨平俊, 戴洋, 陶可欣, 高磊, 杜予州, 曹俊, 俞晓平, 杨倩倩. 长江下游外来生物福寿螺的种类及其种群遗传结构[J]. 生物多样性, 2023, 31(3): 22346-. |
| [2] | 刘勇波. 多倍体植物混合倍性种群的建立机制研究进展[J]. 生物多样性, 2021, 29(8): 1128-1133. |
| [3] | 张梦华, 张宪春. 中国薄叶卷柏复合群的物种划分[J]. 生物多样性, 2021, 29(12): 1607-1619. |
| [4] | 范兴科, 燕霞, 冯媛媛, 冉进华, 钱朝菊, 尹晓月, 周姗姗, 房庭舟, 马小飞. 红砂基因组大小变异及物种分化[J]. 生物多样性, 2021, 29(10): 1308-1320. |
| [5] | 李媛媛, 刘超男, 王嵘, 罗水兴, 农寿千, 王静雯, 陈小勇. 分子标记在濒危物种保护中的应用[J]. 生物多样性, 2020, 28(3): 367-375. |
| [6] | 黄建峰, 徐睿, 彭艳琼. 榕树种间杂交研究进展[J]. 生物多样性, 2019, 27(4): 457-467. |
| [7] | 庄平. 杜鹃花属植物的可育性研究进展[J]. 生物多样性, 2019, 27(3): 327-338. |
| [8] | 胡颖, 王茜, 张新新, 周玮, 陈晓阳, 胡新生. 叶绿体DNA标记在谱系地理学中的应用研究进展[J]. 生物多样性, 2019, 27(2): 219-234. |
| [9] | 莫日根高娃, 商辉, 刘保东, 康明, 严岳鸿. 一个种还是多个种? 简化基因组及其形态学证据揭示中国白桫椤植物的物种多样性分化[J]. 生物多样性, 2019, 27(11): 1196-1204. |
| [10] | 薛晨阳, 许玉凤, 曲波. 不同氮水平下瘤突苍耳、苍耳及其杂交种形态、光合及生长特征比较[J]. 生物多样性, 2018, 26(6): 554-563. |
| [11] | 谢艳萍, 赵建立, 朱兴福, 李莉, 李庆军. 偏花报春和海仙报春3个同域居群的不对称杂交[J]. 生物多样性, 2017, 25(6): 647-653. |
| [12] | 田代科, 李春, 肖艳, 付乃峰, 童毅, 吴瑞娟. 中国秋海棠属植物的自然杂交发生及其特点[J]. 生物多样性, 2017, 25(6): 654-674. |
| [13] | 郑硕理, 田晓玲, 黄承玲, 王灵军, 冯元, 张敬丽. 结合分子手段和形态分析验证大白杜鹃与马缨杜鹃的自然杂交[J]. 生物多样性, 2017, 25(6): 627-637. |
| [14] | 魏宇昆, 黄艳波, 李桂彬. 同域分布共享传粉者的鼠尾草属植物的生殖隔离[J]. 生物多样性, 2017, 25(6): 608-614. |
| [15] | 张小龙, 杨丽华, 康明. 牛耳朵和马坝报春苣苔同域种群授粉后的生殖隔离[J]. 生物多样性, 2017, 25(6): 615-620. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn