



生物多样性 ›› 2017, Vol. 25 ›› Issue (6): 615-620. DOI: 10.17520/biods.2017029 cstr: 32101.14.biods.2017029
收稿日期:2017-02-01
接受日期:2017-04-06
出版日期:2017-06-20
发布日期:2017-07-10
通讯作者:
康明
基金资助:
Xiaolong Zhang1, Lihua Yang1,2, Ming Kang1,*( )
)
Received:2017-02-01
Accepted:2017-04-06
Online:2017-06-20
Published:2017-07-10
Contact:
Kang Ming
摘要:
生殖隔离是物种形成的关键, 也是物种保持完整性和独立性的基础。报春苣苔属(Primulina)是我国苦苣苔科中最大的属, 具有极为丰富的物种多样性。大部分报春苣苔属植物为喀斯特地区特有植物, 近缘种的同域分布也相当普遍。为更好地理解该属植物的物种多样性形成及维持机制, 我们选取了牛耳朵(P. eburnea)和马坝报春苣苔(P. mabaensis)的同域种群, 分析了授粉后的多种隔离机制强度, 主要包括花粉竞争、坐果率、种子重量、种子萌发率、花粉活力。结果显示, 牛耳朵和马坝报春苣苔的授粉后总隔离强度都较弱(0.09 vs. 0.13), 其中花粉竞争及种子萌发率的隔离强度为负值, 对物种间基因流发生起促进作用; 而坐果率、种子重量以及花粉活力的隔离强度均为正值, 表现为对种间基因流起阻止作用。牛耳朵和马坝报春苣苔较弱的授粉后隔离机制不足以完全阻止物种间杂交、保持物种独立性, 但野外较少存在自然杂交个体暗示着两者可能存在较强的授粉前隔离机制。
张小龙, 杨丽华, 康明 (2017) 牛耳朵和马坝报春苣苔同域种群授粉后的生殖隔离. 生物多样性, 25, 615-620. DOI: 10.17520/biods.2017029.
Xiaolong Zhang, Lihua Yang, Ming Kang (2017) Post-pollination reproductive isolation of sympatric populations of Primulina eburnea and P. mabaensis (Gesneriaceae). Biodiversity Science, 25, 615-620. DOI: 10.17520/biods.2017029.
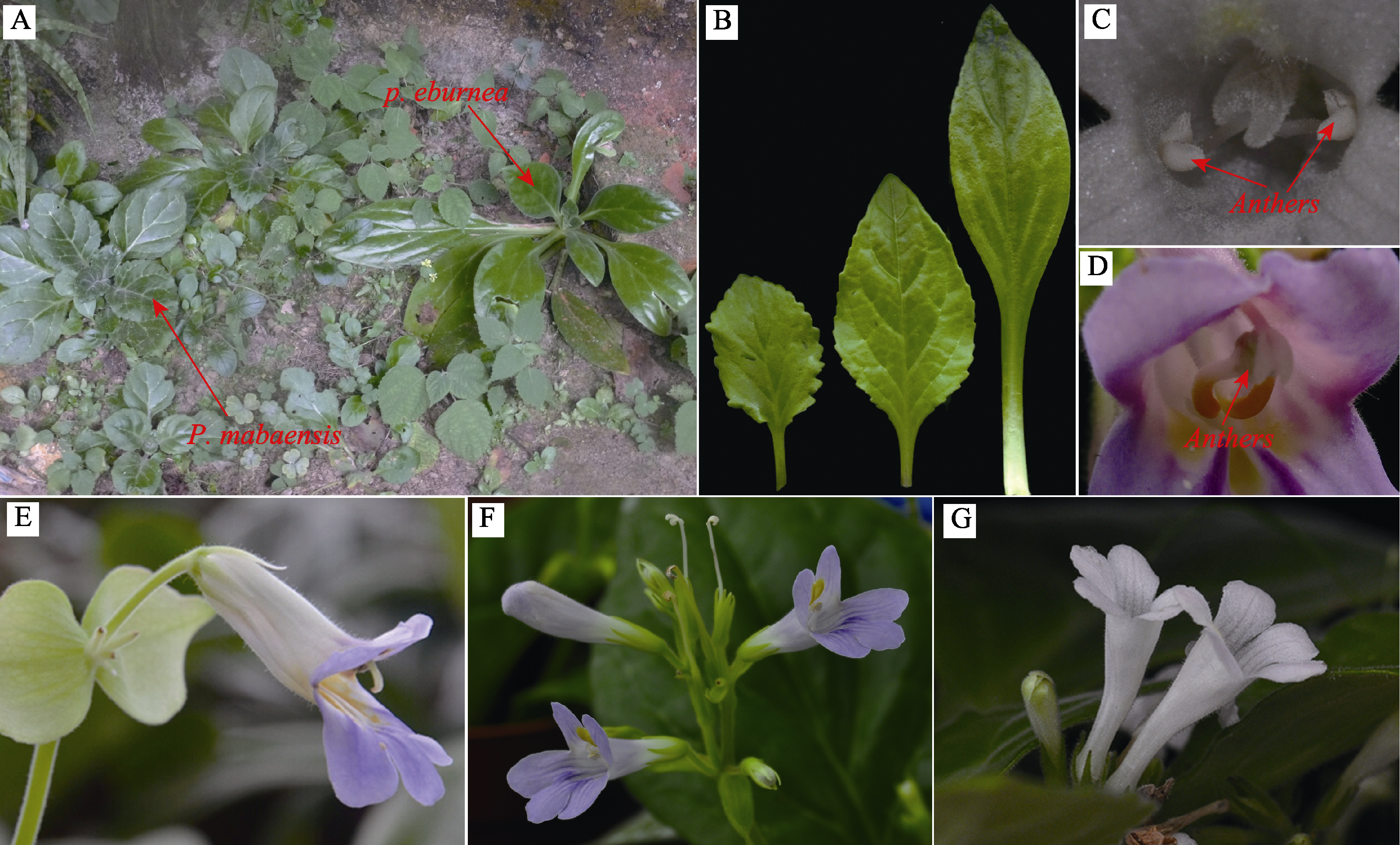
图1 牛耳朵、马坝报春苣苔及其杂交F1代植株、花和叶。(A)牛耳朵和马坝报春苣苔野外植株; (B)牛耳朵(右)、杂交F1代(中)和马坝报春苣苔(左)的叶; (C)马坝报春苣苔花药; (D)牛耳朵花药; (E)牛耳朵花; (F)杂交F1代花; (G)马坝报春苣苔花。
Fig. 1 Plant, flower and leaf of Primulina eburnea, P. mabaensis and their hybrid. (A) Plants of P. eburnea and P. mabaensis in field; (B) Leaves of P. eburnea (right), hybrid (middle) and P. mabaensis (left); (C) Flower of P. mabaensis showing anthers; (D) Flower of P. eburnea showing anthers; (E) Flowers of P. eburnea; (F) Flowers of hybrid; (G) Flowers of P. mabaensis.
| 引物名称 Primer | 正向引物 Forward primer | 反向引物 Reverse primer | 扩增片段长度多态性 Amplified fragment length polymorphism (bp) | |
|---|---|---|---|---|
| 牛耳朵 Primulina eburnea | 马坝报春苣苔 P. mabaensis | |||
| 325 | AACGGAGAACACCCCATTTA | TCGCCTTATGAAGGTTTTGG | 249 | 246 |
| 415 | AACCCATCGTTTCACTCCAC | CTCGGAATCAACTCCTAGCG | 289 | 299 |
| 1143 | CGGAGTCAGCTTTGCACATA | CTCTCTCCTACACACGAGCG | 222 | 219 |
表1 SSR引物信息及扩增长度多态性
Table 1 SSR primer information and amplified fragment length polymorphism
| 引物名称 Primer | 正向引物 Forward primer | 反向引物 Reverse primer | 扩增片段长度多态性 Amplified fragment length polymorphism (bp) | |
|---|---|---|---|---|
| 牛耳朵 Primulina eburnea | 马坝报春苣苔 P. mabaensis | |||
| 325 | AACGGAGAACACCCCATTTA | TCGCCTTATGAAGGTTTTGG | 249 | 246 |
| 415 | AACCCATCGTTTCACTCCAC | CTCGGAATCAACTCCTAGCG | 289 | 299 |
| 1143 | CGGAGTCAGCTTTGCACATA | CTCTCTCCTACACACGAGCG | 222 | 219 |
| 隔离机制 Isolation barriers | 计算方程 Equation for calculating reproductive isolation (RI) |
|---|---|
| 花粉竞争 | 1-2×[异源花粉授粉率/(异源花粉授粉率+同源花粉授粉率)] |
| Pollen competition | 1-2×[interspecific pollination ratio/(interspecific pollination rate + intraspecific pollination rate)] |
| 坐果率 | 1-2×[杂交坐果率/(杂交坐果率+自交坐果率)] |
| Fruit set | 1-2×[interspecific fruit set/(interspecific fruit set + selfing fruit set)] |
| 种子重量 | 1-2×[杂交种子重量/(杂交种子重量+自交种子重量)] |
| Seed mass | 1-2×[interspecific seed mass of per fruit /(interspecific seed mass of per fruit + selfing seed mass of per fruit) ] |
| 种子萌发率 | 1-2×[杂交种子萌发率/(杂交种子萌发率+自交种子萌发率)] |
| Seed germination rate | 1-2×[interspecific seed germination/(interspecific seed germination + selfing seed germination)] |
| 花粉活力 | 1-2×[杂交F1代花粉活力/(杂交F1代花粉活力+亲本花粉活力)] |
| Pollen viability | 1-2×[interspecific F1 pollen viability/(interspecific F1 pollen viability + parent pollen viability)] |
表2 各种生殖隔离机制强度的计算及其变量情况
Table 2 Equations used to quantify components of reproductive isolation. Details of how the variables were constructed are given in the text.
| 隔离机制 Isolation barriers | 计算方程 Equation for calculating reproductive isolation (RI) |
|---|---|
| 花粉竞争 | 1-2×[异源花粉授粉率/(异源花粉授粉率+同源花粉授粉率)] |
| Pollen competition | 1-2×[interspecific pollination ratio/(interspecific pollination rate + intraspecific pollination rate)] |
| 坐果率 | 1-2×[杂交坐果率/(杂交坐果率+自交坐果率)] |
| Fruit set | 1-2×[interspecific fruit set/(interspecific fruit set + selfing fruit set)] |
| 种子重量 | 1-2×[杂交种子重量/(杂交种子重量+自交种子重量)] |
| Seed mass | 1-2×[interspecific seed mass of per fruit /(interspecific seed mass of per fruit + selfing seed mass of per fruit) ] |
| 种子萌发率 | 1-2×[杂交种子萌发率/(杂交种子萌发率+自交种子萌发率)] |
| Seed germination rate | 1-2×[interspecific seed germination/(interspecific seed germination + selfing seed germination)] |
| 花粉活力 | 1-2×[杂交F1代花粉活力/(杂交F1代花粉活力+亲本花粉活力)] |
| Pollen viability | 1-2×[interspecific F1 pollen viability/(interspecific F1 pollen viability + parent pollen viability)] |
| 隔离机制 Isolation barriers | 牛耳朵 P. eburnea | 马坝报春苣苔 P. mabaensis |
|---|---|---|
| 花粉竞争 Pollen competition | -0.321 | -0.026 |
| 坐果率 Fruit set | 0.058 | 0.003 |
| 种子重量 Seed mass | 0.157 | 0.019 |
| 种子萌发率 Seed germination rate | -0.068 | -0.199 |
| 花粉活力 Pollen viability | 0.264 | 0.333 |
| 授粉后隔离总强度 RI | 0.090 | 0.130 |
表3 牛耳朵和马坝报春苣苔授粉后各生殖隔离机制的强度
Table 3 The strength of post-pollination reproductive isolations between Primulina eburnea and P. mabaensis.
| 隔离机制 Isolation barriers | 牛耳朵 P. eburnea | 马坝报春苣苔 P. mabaensis |
|---|---|---|
| 花粉竞争 Pollen competition | -0.321 | -0.026 |
| 坐果率 Fruit set | 0.058 | 0.003 |
| 种子重量 Seed mass | 0.157 | 0.019 |
| 种子萌发率 Seed germination rate | -0.068 | -0.199 |
| 花粉活力 Pollen viability | 0.264 | 0.333 |
| 授粉后隔离总强度 RI | 0.090 | 0.130 |
| [1] | Ai B, Gao Y, Zhong XL, Tao JJ, Kang M, Huang HW (2015) Comparative transcriptome resources of eleven Primulina species, a group of ‘stone plants’ from a biodiversity hot spot. Molecular Ecology Resources, 15, 619-632. |
| [2] | Baack E, Melo MC, Rieseberg LH, Ortiz-Barrientos D (2015) The origins of reproductive isolation in plants. New Phytologist, 207, 968-984. |
| [3] | Brys R, Vanden Broeck A, Mergeay J, Jacquemyn H (2014) The contribution of mating system variation to reproductive isolation in two closely related Centaurium species (Gentianaceae) with a generalized ?ower morphology. Evolution, 68, 1281-1293. |
| [4] | Brys R, Cauwenberghe JV, Jacquemyn H (2016) The importance of autonomous selfing in preventing hybridization in three closely related plant species. Journal of Ecology, 104, 601-610. |
| [5] | Chung KF, Huang HY, Peng JI, Xu WB (2013) Primulina mabaensis (Gesneriaceae), a new species from a limestone cave of northern Guangdong, China. Phytotaxa, 92, 40-48. |
| [6] | Coyne JA, Orr HA (2004) Speciation. Sinauer Associates, Sunderland, MA. |
| [7] | Dafni A (1992) Pollination Biology. Oxford University Press New York. |
| [8] | Gao Y, Ai B, Kong HH, Kang M, Huang HW (2015) Geographical pattern of isolation and diversification in karst habitat islands: a case study in the Primulina eburnea complex. Journal of Biogeography, 42, 2131-2144. |
| [9] | Huang SQ, Shi XQ (2013) Floral isolation in Pedicularis: how do congeners with shared pollinators minimize reproductive interference? New Phytologist, 199, 858-865. |
| [10] | Husband BC, Schemske DW, Burton TL, Goodwillie C (2002) Pollen competition as a unilateral reproductive barrier between sympatric diploid and tetraploid Chamerion angustifolium. Proceedings of the Royal Society B, 269, 2565-2571. |
| [11] | Kang M, Tao JJ, Wang J, Ren C, Qi QW, Xiang QY, Huang HW (2014) Adaptive and nonadaptive genome size evolution in karst endemic flora of China. New Phytologist, 202, 1371-1381. |
| [12] | Kay KM (2006) Reproductive isolation between two closely related humming bird pollinated neotropical gingers. Evolution, 60, 538-552. |
| [13] | Klips RA (1999) Pollen competition as a reproductive isolation mechanism between two sympatric Hibiscus species (Malvaceae). American Journal of Botany, 86, 269-272. |
| [14] | Liu RR, Pan B, Zhou TJ, Liao JP (2012) Cytological studies on Primulina taxa (Gesneriaceae) from limestone karsts in Guangxi Province, China. Caryologia, 65, 295-303. |
| [15] | Luo ZL, Duan TT, Yuan S, Chen S, Bai XF, Zhang DX (2015) Reproductive isolation between sympatric sister species, Mussaenda kwangtungensis and M. pubescens var. alba. Journal of Integrative Plant Biology, 57, 859-870. |
| [16] | Martin NH, Willis JH (2007) Ecological divergence associated with mating system causes nearly complete reproductive isolation between sympatric Mimulus species. Evolution, 61, 68-82. |
| [17] | Möller M, Wei YG, Wen F, Clark JL, Weber A (2016) You win some you lose some: updated generic delineations and classification of Gesneriaceae—implications for the family in China.Guihaia, 36, 44-60. |
| [18] | Rhymer JM, Simberloff D (1996) Extinction by hybridization and introgression. Annual Review of Ecology, Evolution and Systematics, 27, 83-109. |
| [19] | Snow AA, Spira TP (1996) Pollen-tube competition and male fitness in Hibiscus moscheutos. Evolution, 50, 1866-1870. |
| [20] | Sobel JM, Chen GF (2014) Unification of methods for estimating the strength of reproductive isolation. Evolution, 68, 1511-1522. |
| [21] | Soltis PS, Soltis DE (2009) The role of hybridization in plant speciation. Annual Review of Plant Biology, 60, 561-588. |
| [22] | Wang WT, Pan KY, Li ZY, Weitzman AL, Skog LE (1998) Gesneriaceae. In: Flora of China (eds Wu ZY, Raven PH), pp. 244-401. Science Press, Beijing & Missouri Botanical Garden Press, St. Louis. |
| [23] | Wolf DE, Takebayashi N, Riesebrg LH (2001) Predicting the risk of extinction through hybridization. Conservation Biology, 15, 1039-1053. |
| [24] | Xu SQ, Schlüter PM, Scopece G, Breitkopf H, Gross K, Cozzolino S, Schiestl FP (2011) Floral isolation is the main reproductive barrier among closely related sexually deceptive orchids. Evolution, 65, 2606-2620. |
| [1] | 蒲佳佳, 杨平俊, 戴洋, 陶可欣, 高磊, 杜予州, 曹俊, 俞晓平, 杨倩倩. 长江下游外来生物福寿螺的种类及其种群遗传结构[J]. 生物多样性, 2023, 31(3): 22346-. |
| [2] | 范兴科, 燕霞, 冯媛媛, 冉进华, 钱朝菊, 尹晓月, 周姗姗, 房庭舟, 马小飞. 红砂基因组大小变异及物种分化[J]. 生物多样性, 2021, 29(10): 1308-1320. |
| [3] | 李媛媛, 刘超男, 王嵘, 罗水兴, 农寿千, 王静雯, 陈小勇. 分子标记在濒危物种保护中的应用[J]. 生物多样性, 2020, 28(3): 367-375. |
| [4] | 黄建峰, 徐睿, 彭艳琼. 榕树种间杂交研究进展[J]. 生物多样性, 2019, 27(4): 457-467. |
| [5] | 庄平. 杜鹃花属植物的可育性研究进展[J]. 生物多样性, 2019, 27(3): 327-338. |
| [6] | 胡颖, 王茜, 张新新, 周玮, 陈晓阳, 胡新生. 叶绿体DNA标记在谱系地理学中的应用研究进展[J]. 生物多样性, 2019, 27(2): 219-234. |
| [7] | 莫日根高娃, 商辉, 刘保东, 康明, 严岳鸿. 一个种还是多个种? 简化基因组及其形态学证据揭示中国白桫椤植物的物种多样性分化[J]. 生物多样性, 2019, 27(11): 1196-1204. |
| [8] | 梁思琪, 张宪春, 卫然. 利用整合分类学方法进行蕨类植物复合体的物种划分: 以线裂铁角蕨复合体为例[J]. 生物多样性, 2019, 27(11): 1205-1220. |
| [9] | 薛晨阳, 许玉凤, 曲波. 不同氮水平下瘤突苍耳、苍耳及其杂交种形态、光合及生长特征比较[J]. 生物多样性, 2018, 26(6): 554-563. |
| [10] | 谢艳萍, 赵建立, 朱兴福, 李莉, 李庆军. 偏花报春和海仙报春3个同域居群的不对称杂交[J]. 生物多样性, 2017, 25(6): 647-653. |
| [11] | 田代科, 李春, 肖艳, 付乃峰, 童毅, 吴瑞娟. 中国秋海棠属植物的自然杂交发生及其特点[J]. 生物多样性, 2017, 25(6): 654-674. |
| [12] | 郑硕理, 田晓玲, 黄承玲, 王灵军, 冯元, 张敬丽. 结合分子手段和形态分析验证大白杜鹃与马缨杜鹃的自然杂交[J]. 生物多样性, 2017, 25(6): 627-637. |
| [13] | 魏宇昆, 黄艳波, 李桂彬. 同域分布共享传粉者的鼠尾草属植物的生殖隔离[J]. 生物多样性, 2017, 25(6): 608-614. |
| [14] | 李霖锋, 刘宝. 表观遗传变异在植物杂交与多倍化过程中的作用[J]. 生物多样性, 2017, 25(6): 600-607. |
| [15] | 王玉国. 自然杂交与物种形成[J]. 生物多样性, 2017, 25(6): 565-576. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn