



Biodiv Sci ›› 2021, Vol. 29 ›› Issue (8): 1128-1133. DOI: 10.17520/biods.2021213 cstr: 32101.14.biods.2021213
• Reviews • Previous Articles Next Articles
Received:2021-05-25
Accepted:2021-07-20
Online:2021-08-20
Published:2021-08-16
Contact:
Yongbo Liu
Yongbo Liu. The mechanism of constructing mixed-ploidy populations in polyploid species[J]. Biodiv Sci, 2021, 29(8): 1128-1133.
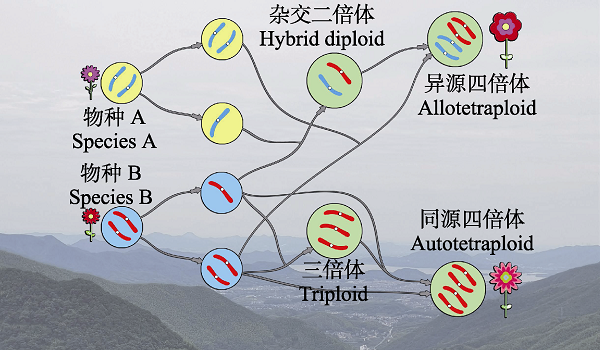
Fig. 1 Formation paths to polyploid species. Diploid species produce normal haploid gametes and unreduced gametes. Unreduced gametes combine with haploid gametes to give rise to triploids or fuse to be autotetraploid species. Diploids can yield somatically polyploids. Allotetraploids are yielded through the fusion of unreduced gametes or the hybridization of haploid gametes from different species.
| [1] |
Arrigo N, Barker MS (2012) Rarely successful polyploids and their legacy in plant genomes. Current Opinion in Plant Biology, 15, 140-146.
DOI PMID |
| [2] | Babcock EB, Stebbins GL (1938) The American Species of Crepis: Their Interrelationships and Distribution as Affected by Polyploidy and Apomixis. Carnegie Institution of Washington Publication, no. 504, Washington, USA. |
| [3] |
Baduel P, Bray S, Vallejo-Marin M, Kolář F, Yant L (2018) The “Polyploid Hop”: Shifting challenges and opportunities over the evolutionary lifespan of genome duplications. Frontiers in Ecology and Evolution, 6, 117.
DOI URL |
| [4] |
Baniaga AE, Marx HE, Arrigo N, Barker MS (2020) Polyploid plants have faster rates of multivariate niche differentiation than their diploid relatives. Ecology Letters, 23, 68-78.
DOI URL |
| [5] |
Blaine Marchant D, Soltis DE, Soltis PS (2016) Patterns of abiotic niche shifts in allopolyploids relative to their progenitors. New Phytologist, 212, 708-718.
DOI PMID |
| [6] |
Bottani S, Zabet NR, Wendel JF, Veitia RA (2018) Gene Expression dominance in allopolyploids: Hypotheses and models. Trends in Plant Science, 23, 393-402.
DOI PMID |
| [7] |
Castro M, Loureiro J, Figueiredo A, Serrano M, Husband BC, Castro S (2020) Different patterns of ecological divergence between two tetraploids and their diploid counterpart in a parapatric linear coastal distribution polyploid complex. Frontiers in Plant Science, 11, 315.
DOI URL |
| [8] | De Smet R, Adams KL, Vandepoele K, Van Montagu MCE, Maere S, Van de Peer Y (2013) Convergent gene loss following gene and genome duplications creates single-copy families in flowering plants. Proceedings of the National Academy of Sciences, USA, 110, 2898-2903. |
| [9] |
Doyle JJ, Coate JE (2019) Polyploidy, the nucleotype, and novelty: The impact of genome doubling on the biology of the cell. International Journal of Plant Sciences, 180, 1-52.
DOI URL |
| [10] |
Eric Schranz M, Mohammadin S, Edger PP (2012) Ancient whole genome duplications, novelty and diversification: The WGD Radiation Lag-Time Model. Current Opinion in Plant Biology, 15, 147-153.
DOI PMID |
| [11] | Fawcett JA, Maere S, Van de Peer Y (2009) Plants with double genomes might have had a better chance to survive the Cretaceous-Tertiary extinction event. Proceedings of the National Academy of Sciences, USA, 106, 5737-5742. |
| [12] | Folk RA, Siniscalchi CM, Soltis DE (2020) Angiosperms at the edge: Extremity, diversity, and phylogeny. Plant, Cell & Environment, 43, 2871-2893. |
| [13] |
Freeling M (2017) Picking up the Ball at the K/Pg Boundary: The distribution of ancient polyploidies in the plant phylogenetic tree as a spandrel of asexuality with occasional sex. The Plant Cell, 29, 202-206.
DOI PMID |
| [14] |
Gunn BF, Murphy DJ, Walsh NG, Conran JG, Pires JC, MacFarlane TD, Birch JL (2020) Evolution of Lomandroideae: Multiple origins of polyploidy and biome occupancy in Australia. Molecular Phylogenetics and Evolution, 149, 106836.
DOI PMID |
| [15] |
Hias N, Svara A, Keulemans JW (2018) Effect of polyploidisation on the response of apple (Malus ×domestica Borkh.) toVenturia inaequalis infection. European Journal of Plant Pathology, 151, 515-526.
DOI URL |
| [16] |
Hohmann N, Wolf EM, Lysak MA, Koch MA (2015) A time-calibrated road map of Brassicaceae species radiation and evolutionary history. The Plant Cell, 27, 2770-2784.
DOI PMID |
| [17] |
Hülber K, Sonnleitner M, Suda J, Krejčíková J, Schönswetter P, Schneeweiss GM, Winkler M (2015) Ecological differentiation, lack of hybrids involving diploids, and asymmetric gene flow between polyploids in narrow contact zones ofSenecio carniolicus (syn. Jacobaea carniolica, Asteraceae). Ecology and Evolution, 5, 1224-1234.
DOI URL |
| [18] |
Jiao YN, Wickett NJ, Ayyampalayam S, Chanderbali AS, Landherr L, Ralph PE, Tomsho LP, Hu Y, Liang HY, Soltis PS, Soltis DE, Clifton SW, Schlarbaum SE, Schuster SC, Ma H, Leebens-Mack J, de Pamphilis CW (2011) Ancestral polyploidy in seed plants and angiosperms. Nature, 473, 97-100.
DOI URL |
| [19] |
Kao RH (2007) Asexuality and the coexistence of cytotypes. New Phytologist, 175, 764-772.
DOI URL |
| [20] |
Kolář F, Čertner M, Suda J, Schönswetter P, Husband BC (2017) Mixed-ploidy species: Progress and opportunities in polyploid research. Trends in Plant Science, 22, 1041-1055.
DOI URL |
| [21] |
Levin DA (1975) Minority cytotype exclusion in local plant populations. Taxon, 24, 35-43.
DOI URL |
| [22] |
Li DW, Liu YF, Zhong CH, Huang HW (2010) Morphological and cytotype variation of wild kiwifruit (Actinidia chinensis complex) along an altitudinal and longitudinal gradient in central-west China. Botanical Journal of the Linnean Society, 164, 72-83.
DOI URL |
| [23] |
Liang SQ, Zhang XC, Wei R (2019) Integrative taxonomy resolved species delimitation in a fern complex: A case study of theAsplenium coenobiale complex. Biodiversity Science, 27, 1205-1220. (in Chinese with English abstract)
DOI |
|
[ 梁思琪, 张宪春, 卫然 (2019) 利用整合分类学方法进行蕨类植物复合体的物种划分: 以线裂铁角蕨复合体为例. 生物多样性, 27, 1205-1220.]
DOI |
|
| [24] |
Mandák B, Vít P, Krak K, Trávníček P, Havrdová A, Hadincová V, Zákravský P, Jarolímová V, Bacles CFE, Douda J (2016) Flow cytometry, microsatellites and niche models reveal the origins and geographical structure ofAlnus glutinosa populations in Europe. Annals of Botany, 117, 107-120.
DOI URL |
| [25] |
Mayrose I, Zhan SH, Rothfels CJ, Magnuson-Ford K, Barker MS, Rieseberg LH, Otto SP (2011) Recently formed polyploid plants diversify at lower rates. Science, 333, 1257-1257.
DOI PMID |
| [26] | Morgan C, Zhang HK, Henry CE, Franklin FCH, Bomblies K (2020) Derived alleles of two axis proteins affect meiotic traits in autotetraploidArabidopsis arenosa. Proceedings of the National Academy of Sciences, USA, 117, 8980-8988. |
| [27] |
Mráz P, Španiel S, Keller A, Bowmann G, Farkas A, Šingliarová B, Rohr RP, Broennimann O, Müller-Schärer H (2012) Anthropogenic disturbance as a driver of microspatial and microhabitat segregation of cytotypes ofCentaurea stoebe and cytotype interactions in secondary contact zones. Annals of Botany, 110, 615-627.
DOI URL |
| [28] |
Nuismer SL, Cunningham BM (2005) Selection for phenotypic divergence between diploid and autotetraploidHeuchera grossulariifolia. Evolution, 59, 1928-1935.
PMID |
| [29] |
Ramsey J, Ramsey TS (2014) Ecological studies of polyploidy in the 100 years following its discovery. Philosophical Transactions of the Royal Society B: Biological Sciences, 369, 20130352.
DOI URL |
| [30] |
Ramsey J, Schemske DW (1998) Pathways, mechanisms, and rates of polyploid formation in flowering plants. Annual Review of Ecology and Systematics, 29, 467-501.
DOI URL |
| [31] | Rao JY, Liu YF, Huang HW (2012) Analysis of ploidy segregation and genetic variation of progenies of different interploidy crosses inActinidia chinensis. Acta Horticulturae Sinica, 39, 1447-1456. (in Chinese with English abstract) |
| [ 饶静云, 刘义飞, 黄宏文 (2012) 中华猕猴桃不同倍性间杂交后代倍性分离和遗传变异分析. 园艺学报, 39, 1447-1456.] | |
| [32] |
Ren R, Wang HF, Guo CC, Zhang N, Zeng LP, Chen YM, Ma H, Qi J (2018) Widespread whole genome duplications contribute to genome complexity and species diversity in angiosperms. Molecular Plant, 11, 414-428.
DOI PMID |
| [33] |
Rice A, Glick L, Abadi S, Einhorn M, Kopelman NM, Salman-Minkov A, Mayzel J, Chay O, Mayrose I (2015) The Chromosome Counts Database (CCDB)—A community resource of plant chromosome numbers. New Phytologist, 206, 19-26.
DOI URL |
| [34] | Rice A, Šmarda P, Novosolov M, Drori M, Glick L, Sabath N, Meiri S, Belmaker J, Mayrose I (2019) The global biogeography of polyploid plants. Nature Ecology & Evolution, 3, 265-273. |
| [35] |
Rieseberg LH, Willis JH (2007) Plant speciation. Science, 317, 910-914.
PMID |
| [36] |
Sabara HA, Kron P, Husband BC (2013) Cytotype coexistence leads to triploid hybrid production in a diploid-tetraploid contact zone ofChamerion angustifolium (Onagraceae). American Journal of Botany, 100, 962-970.
DOI PMID |
| [37] |
Soltis DE, Albert VA, Leebens-Mack J, Bell CD, Paterson AH, Zheng CF, Sankoff Dde Pamphilis CW, Wall PK, Soltis PS (2009) Polyploidy and angiosperm diversification. American Journal of Botany, 96, 336-348.
DOI URL |
| [38] |
Soltis DE, Segovia-Salcedo MC, Jordon-Thaden I, Majure L, Miles NM, Mavrodiev EV, Mei WB, Cortez MB, Soltis PS, Gitzendanner MA (2014) Are polyploids really evolutionary dead-ends (again)? A critical reappraisal of Mayrose et al (2011). New Phytologist, 202, 1105-1117.
DOI URL |
| [39] |
Sonnleitner M, Hülber K, Flatscher R, García PE, Winkler M, Suda J, Schönswetter P, Schneeweiss GM (2016) Ecological differentiation of diploid and polyploid cytotypes ofSenecio carniolicus sensu lato (Asteraceae) is stronger in areas of sympatry. Annals of Botany, 117, 269-276.
DOI PMID |
| [40] |
Stevens AV, Nicotra AB, Godfree RC, Guja LK (2020) Polyploidy affects the seed, dormancy and seedling characteristics of a perennial grass, conferring an advantage in stressful climates. Plant Biology, 22, 500-513.
DOI PMID |
| [41] | Stebbins GL (1950) Variation and Evolution in Plants. Columbia University Press, New York. |
| [42] | Stebbins GL (1971) Chromosomal Evolution in Higher Plants. Edward Arnold, London. |
| [43] |
Suda J, Weiss-Schneeweiss H, Tribsch A, Schneeweiss GM, Trávníček P, Schönswetter P (2007) Complex distribution patterns of di-, tetra-, and hexaploid cytotypes in the European high mountain plantSenecio carniolicus (Asteraceae). American Journal of Botany, 94, 1391-1401.
DOI PMID |
| [44] |
Sutherland BL, Galloway LF (2017) Postzygotic isolation varies by ploidy level within a polyploid complex. New Phytologist, 213, 404-412.
DOI PMID |
| [45] |
The Brassica rapa Genome Sequencing Project Consortium (2011) The genome of the mesopolyploid crop speciesBrassica rapa. Nature Genetics, 43, 1035-1039.
DOI URL |
| [46] |
Van de Peer Y, Ashman TL, Soltis PS, Soltis DE (2021) Polyploidy: An evolutionary and ecological force in stressful times. The Plant Cell, 33, 11-26.
DOI URL |
| [47] |
Van de Peer Y, Mizrachi E, Marchal K (2017) The evolutionary significance of polyploidy. Nature Reviews Genetics, 18, 411-424.
DOI URL |
| [48] |
Wang WN, He YH, Cao Z, Deng ZN (2018) Induction of tetraploids inImpatiens (Impatiens walleriana) and characterization of their changes in morphology and resistance to downy mildew. HortScience, 53, 925-931.
DOI URL |
| [49] | Wood TE, Takebayashi N, Barker MS, Mayrose I, Greenspoon PB, Rieseberg LH (2009) The frequency of polyploid speciation in vascular plants. Proceedings of the National Academy of Sciences, USA, 106, 13875-13879. |
| [50] |
Wu SD, Han BC, Jiao YN (2020) Genetic contribution of paleopolyploidy to adaptive evolution in angiosperms. Molecular Plant, 13, 59-71.
DOI URL |
| [51] |
Yant L, Hollister JD, Wright KM, Arnold BJ, Higgins JD, Franklin FCH, Bomblies K (2013) Meiotic adaptation to genome duplication inArabidopsis arenosa. Current Biology, 23, 2151-2156.
DOI URL |
| [52] | Zeng H, Li DW, Huang HW (2009) Distribution pattern of ploidy variation ofActinidia chinensis andA. deliciosa. Journal of Wuhan Botanical Research, 27, 312-317. (in Chinese with English abstract) |
| [ 曾华, 李大卫, 黄宏文 (2009) 中华猕猴桃和美味猕猴桃的倍性变异及地理分布研究. 武汉植物学研究, 27, 312-317.] | |
| [53] | Zhang HK, Bian Y, Gou XW, Dong YZ, Rustgi S, Zhang BJ, Xu CM, Li N, Qi B, Han FP, von Wettstein D, Liu B (2013) Intrinsic karyotype stability and gene copy number variations may have laid the foundation for tetraploid wheat formation. Proceedings of the National Academy of Sciences, USA, 110, 19466-19471. |
| [54] |
Zhang K, Wang XW, Cheng F (2019) Plant polyploidy: Origin, evolution, and its influence on crop domestication. Horticultural Plant Journal, 5, 231-239.
DOI |
| [1] | Zheming Sun, Yaheng Liu, Qiutong Peng, Zhiyan Xu, Yujing Yang, Wenhui Ou, Zhongqiang Li. Competition status and conservation suggestions for Wild Plant with Extremely Small Populations in primary communities in Hubei Province [J]. Biodiv Sci, 2022, 30(6): 21517-. |
| [2] | Zhiyong Yuan, Jinmin Chen, Yunhe Wu, Xianqi Li, Jing Che. Revision of the list of amphibian species in Yunnan Province [J]. Biodiv Sci, 2022, 30(4): 21470-. |
| [3] | Yu Ren, Shengli Tao, Tianyu Hu, Haitao Yang, Hongcan Guan, Yanjun Su, Kai Cheng, Mengxi Chen, Huawei Wan, Qinghua Guo. The outlook and system construction for monitoring Essential Biodiversity Variables based on remote sensing: The case of China [J]. Biodiv Sci, 2022, 30(10): 22530-. |
| [4] | Yue Xu, Runguo Zang. Theoretical and practical research on conservation of Wild Plants with Extremely Small Populations in China [J]. Biodiv Sci, 2022, 30(10): 22505-. |
| [5] | Zhi Yao, Jun Guo, Chenzhong Jin, Yongbo Liu. Endangered mechanisms for the first-class protected Wild Plants with Extremely Small Populations in China [J]. Biodiv Sci, 2021, 29(3): 394-408. |
| [6] | Qiuhong Feng, Dengfeng Li, Tao Yu, Junqing Li, Wenbao Ma, Lei Zhang. Phenotypic fruit and seed variations of Acer catalpifolium, a Wild Plant with Extremely Small Populations in China [J]. Biodiv Sci, 2020, 28(3): 314-322. |
| [7] | Sha Deng, Yanni Wu, Kunlin Wu, Lin Fang, Lin Li, Songjun Zeng. Breeding characteristics and artificial propagation of 14 species of Wild Plant with Extremely Small Populations (WPESP) in China [J]. Biodiv Sci, 2020, 28(3): 385-400. |
| [8] | Dongdong Chen, Zhenqing Li. Population viability analysis of Wild Plant with Extremely Small Populations (WPESP): Methods, problems and prospects [J]. Biodiv Sci, 2020, 28(3): 358-366. |
| [9] | Zhixia Zhao, Changming Zhao, Shuyu Deng, Guozhen Shen, Zongqiang Xie, Gaoming Xiong, Junqing Li. Community structure and dynamics of a remnant forest dominated by Thuja sutchuenensis after deforestation [J]. Biodiv Sci, 2020, 28(3): 333-339. |
| [10] | Xinghui Lu, Runguo Zang, Yi Ding, Jihong Huang, Yue Xu. Habitat characteristics and its effects on seedling abundance of Hopea hainanensis, a Wild Plant with Extremely Small Populations [J]. Biodiv Sci, 2020, 28(3): 289-295. |
| [11] | Jinyuan Su, Yu Yan, Chong Li, Dan Li, Fang K. Du. Informing conservation strategies with genetic diversity in Wild Plant with Extremely Small Populations: A review on gymnosperms [J]. Biodiv Sci, 2020, 28(3): 376-384. |
| [12] | Shitong Wang, Yaozhan Xu, Teng Yang, Xinzeng Wei, Mingxi Jiang. Impacts of microhabitats on leaf functional traits of the wild population of Sinojackia huangmeiensis [J]. Biodiv Sci, 2020, 28(3): 277-288. |
| [13] | Yaobin Song, Li Xu, Junpeng Duan, Weijun Zhang, Xiaolu Shentu, Tianxiang Li, Runguo Zang, Ming Dong. Sex ratio and spatial pattern of Taxus fuana, a Wild Plant with Extremely Small Populations in Tibet [J]. Biodiv Sci, 2020, 28(3): 269-276. |
| [14] | Leilei Yang,Wenguang Wang,Xiaoan Lang,Suzhou Zhang,Zhangxiu Yao,Ting Xu,Yuanqiu Li,Danfeng Yan,Jianfen Yang,Yaling Wang,Shouzhou Zhang. Resource status of Michelia guangdongensis (Magnoliaceae), a wild plant species with extremely small populations [J]. Biodiv Sci, 2019, 27(9): 1016-1020. |
| [15] | Wang Shitong, Wu Hao, Liu Mengting, Zhang Jiaxin, Liu Jianming, Meng Hongjie, Xu Yaozhan, Qiao Xiujuan, Wei Xinzeng, Lu Zhijun, Jiang Mingxi. Community structure and dynamics of a remnant forest dominated by a plant species with extremely small population (Sinojackia huangmeiensis) in central China [J]. Biodiv Sci, 2018, 26(7): 749-759. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2026 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
