



Biodiv Sci ›› 2013, Vol. 21 ›› Issue (1): 111-116. DOI: 10.3724/SP.J.1003.2013.06187 cstr: 32101.14.SP.J.1003.2013.06187
• Orginal Article • Previous Articles Next Articles
Caroline A. Polgar, Richard B. Primack*( )
)
Received:2012-10-08
Accepted:2012-12-27
Online:2013-01-20
Published:2013-02-04
Contact:
B. Primack Richard
Caroline A. Polgar, Richard B. Primack. Leaf out phenology in temperate forests[J]. Biodiv Sci, 2013, 21(1): 111-116.

Fig. 1 Leaf out pictures taken by scientists monitoring leafing in a traditional on-the-ground study (taken by Richard B. Primack at the Arnold Arboretum in Boston, Massachusetts USA)
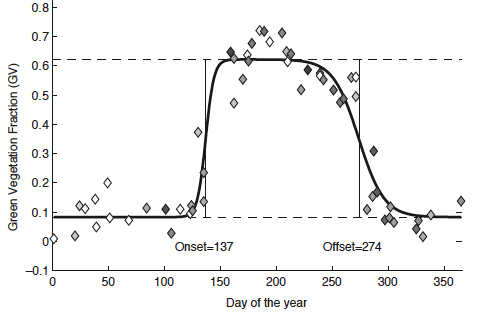
Fig. 2 A graph showing the growth of the leaf canopy over a growing seasons using satellite data from a location in New England, in the northeastern United States. The vegetation data is fit to logistic growth sigmoid functions and the onset and offset of greenness are calculated at the half-maxima of the curve. The quality of the data points is indicated by the symbol shading, with black diamonds having the least error and white diamonds having the most error. Figure from Fisher et al., 2006).

Fig. 3 A sequence of photos taken over a three week period in the spring of 2011 showing the development of the leaf canopy at Minute Man National Historical Site, a park in Concord, Massachusetts (Photos by Richard B. Primack)

Fig. 4 A hillside in the northeastern United States showing the effects of a late frost following a period of warming. Trees that responded more quickly to warm temperatures, such as sugar maples, suffered damage to early leaves, while more conservative leafing species, such as American beech, fared better and are developing normally. Figure from (Hufkens et al., 2012b).

Fig. 5 Leaf out pictures taken at the Arnold Arboretum in Boston, Massachusetts USA by scientists monitoring leafing in a traditional on-the-ground study (taken by Dr. Richard B. Primack)
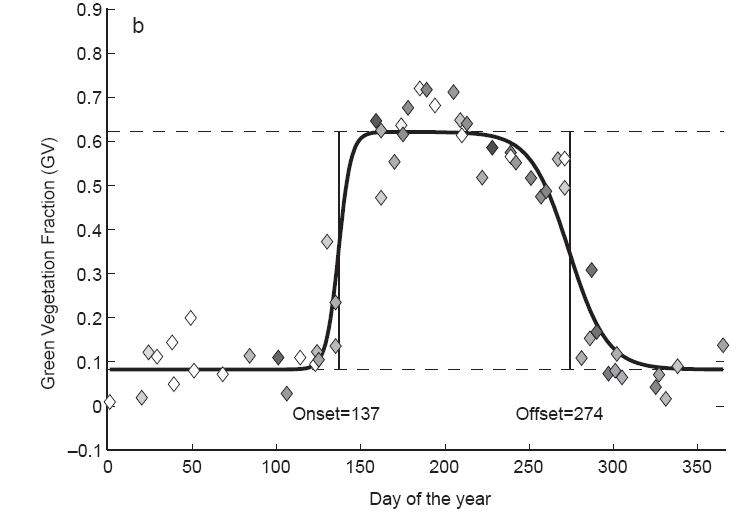
Fig. 6 A graph showing the growth of the leaf canopy over a growing season using satellite data from a location in New England, in the northeastern United States. The vegetation data is fit to logistic growth sigmoid functions and the onset and offset of greenness are calculated at the half-maxima of the curve. The quality of the data points is indicated by the symbol shading, with black diamonds having the least error and white diamonds having the most error (Figure from Fisher et al., 2006).

Fig. 7 A sequence of photos taken over a three week period in the spring of 2011 showing the development of the leaf canopy at Minute Man National Historical Site, a park in Concord, Massachusetts, with a bridge, a monument, and statue as points of reference. The leaf out times of individual trees can be seen in these photos. Photos by Richard B. Primack.

Fig. 8 A hillside in the northeastern United States showing the effects of a late frost following a period of warming. Trees that responded more quickly to warm temperatures, such as sugar maples, suffered damage to early leaves, while more conservative leafing species, such as American beech, fared better and are developing normally. Figure from (Hufkens et al., 2012b)
| 1 | Ahl DE, Gower ST, Burrows SN, Shabanov NV, Myneni RB, Knyazikhin Y (2006) Monitoring spring canopy phenology of a deciduous broadleaf forest using MODIS.Remote Sensing of Environment, 104, 88-95. |
| 2 | Bartomeus I, Ascher JS, Wagner D, Danforth BN, Colla S, Kornbluth S, Winfree R (2011) Climate-associated phenological advances in bee pollinators and bee-pollinated plants.Proceedings of the National Academy of Sciences, USA, 108, 20645-20649. |
| 3 | Both C, van Asch M, Bijlsma RG, van den Burg AB, Visser ME (2009) Climate change and unequal phenological changes across four trophic levels: constraints or adaptations?Journal of Animal Ecology, 78, 73-83. |
| 4 | Bradley NL, Leopold AC, Ross J, Huffaker W (1999) Phenological changes reflect climate change in Wisconsin.Proceedings of the National Academy of Sciences, USA, 96, 9701-9704. |
| 5 | Caffarra A, Donnelly A (2010) The ecological significance of phenology in four different tree species: effects of light and temperature on bud burst.International Journal of Biometeorology, DOI:10.1007/s00484-00010-00386-00481. |
| 6 | Cleland EE, Chuine I, Menzel A, Mooney HA, Schwartz MD (2007) Shifting plant phenology in response to global change.Trends in Ecology and Evolution, 22, 357-365. |
| 7 | Coyle DR, Jordan MS, Raffa KF (2010) Host plant phenology affects performance of an invasive weevil, Phyllobius oblongus (Coleoptera: Curculionidae), in a northern hardwood forest.Environmental Entomology, 39, 1539-1544. |
| 8 | Crimmins MA, Crimmins TM (2008) Monitoring plant phenology using digital repeat photography.Environmental Management, 41, 949-958. |
| 9 | Delbart N, Picard G, Le Toans T, Kergoat L, Quegan S, Woodward I, Dye D, Fedotova V (2008) Spring phenology in boreal Eurasia over a nearly century time scale.Global Change Biology, 14, 603-614. |
| 10 | Egusa S, Nishida T, Fujisaki K, Sawada H (2006) Spatio-temporal abundance of flushing leaves shapes host selection in the willow leaf beetle, Plagiodera versicolora.Entomologia Experimentalis et Applicata, 120, 229-237. |
| 11 | Farmer RE (1968) Sweetgum dormancy release: effects of chilling photoperiod and genotype.Physiologia Plantarum, 21, 1241-1248. |
| 12 | Fisher JI, Mustard JF, Vadeboncoeur MA (2006) Green leaf phenology at Landsat resolution: scaling from the field to the satellite.Remote Sensing of Environment, 100, 265-279. |
| 13 | Forrest J, Miller-Rushing AJ (2010) Toward a synthetic understanding of the role of phenology in ecology and evolution.Philosophical Transactions of the Royal Society B—Biological Sciences, 365, 3101-3112. |
| 14 | Fridley JD (2012) Extended leaf phenology and the autumn niche in deciduous forest invasions.Nature, 485, 359-362. |
| 15 | Ghelardini L, Santini A, Black-Samuelsson S, Myking T, Falusi M (2010) Bud dormancy release in elm (Ulmus spp) clones: a case study of photoperiod and temperature responses.Tree Physiology, 30, 264-274. |
| 16 | Gonsamo A, Chen JM, Wu CY, Dragoni D (2012) Predicting deciduous forest carbon uptake phenology by upscaling FLUXNET measurements using remote sensing data.Agricultural and Forest Meteorology, 165, 127-135. |
| 17 | Gu LH, Hanson PJ, Mac Post W, Kaiser DP, Yang B, Nemani R, Pallardy SG, Meyers T (2008) The 2007 eastern US spring freeze: increased cold damage in a warming world?BioScience, 58, 253-262. |
| 18 | Harrington RA, Brown BJ, Reich PB (1989) Ecophysiology of exotic and native shrubs in southern Wisconsin 1. Relationship of leaf characteristics, resource availability, and phenology to seasonal patterns of carbon gain.Oecologia, 80, 356-367. |
| 19 | Hufkens K, Friedl M, Sonnentag O, Braswell BH, Milliman T, Richardson AD (2012a) Linking near-surface and satellite remote sensing measurements of deciduous broadleaf forest phenology.Remote Sensing of Environment, 117, 307-321. |
| 20 | Hufkens K, Friedl MA, Keenan TF, Sonnentag O, Bailey A, O'Keefe J, Richardson AD (2012b) Ecological impacts of a widespread frost event following early spring leaf-out.Global Change Biology, 18, 2365-2377. |
| 21 | Hunter AF, Lechowicz MJ (1992) Predicting the timing of budburst in temperate trees.Journal of Applied Ecology, 29, 597-604. |
| 22 | Ibáñez I, Primack RB, Miller-Rushing AJ, Ellwood E, Higuchi H, Lee SD, Kobori H, Silander JA (2010) Forecasting phenology under global warming.Philosophical Transactions of the Royal Society B—Biological Sciences, 365, 3247-3260. |
| 23 | Ide R, Oguma H (2010) Use of digital cameras for phenological observations.Ecological Informatics, 5, 339-347. |
| 24 | Körner C, Basler D (2010) Phenology under global warming.Science, 327, 1461-1462. |
| 25 | Linkosalo T, Häkkinen R, Hänninen H (2006) Models of the spring phenology of boreal and temperate trees: Is there something missing?Tree Physiology, 26, 1165-1172. |
| 26 | Menzel A (2000) Trends in phenological phases in Europe between 1951 and 1996.International Journal of Biometeorology, 44, 76-81. |
| 27 | Miller-Rushing AJ, Lloyd-Evans TL, Primack RB, Satzinger P (2008) Bird migration times, climate change, and changing population sizes.Global Change Biology, 14, 1959-1972. |
| 28 | Morin X, Lechowicz MJ, Augspurger C, O' Keefe J, Viner D, Chuine I (2009) Leaf phenology in 22 North American tree |
| 29 | species during the 21st century.Global Change Biology, 15, 961-975. |
| 30 | Partanen J (2004) Dependence of photoperiodic response of growth cessation on the stage of development in Picea abies and Betula pendula seedlings.Forest Ecology and Management, 188, 137-148. |
| 31 | Perry TO (1971) Dormancy of trees in winter.Science, 171, 29-36. |
| 32 | Pettorelli N, Vik JO, Mysterud A, Gaillard JM, Tucker CJ, Stenseth NC (2005) Using the satellite-derived NDVI to assess ecological responses to environmental change.Trends in Ecology and Evolution, 20, 503-510. |
| 33 | Polgar CA, Primack RB (2011) Leaf-out phenology of temperate woody plants: from trees to ecosystems.New Phytologist, 191, 926-941. |
| 34 | Primack RB, Miller-Rushing AJ (2012) Uncovering, collecting, and analyzing records to investigate the ecological impacts of climate change: a template from Thoreau's Concord.BioScience, 62, 170-181. |
| 35 | Reed BC, Brown JF, Vanderzee D, Loveland TR, Merchant JW, Ohlen DO (1994) Measuring phenological variability from satellite imagery.Journal of Vegetation Science, 5, 703-714. |
| 36 | Richardson AD, Bailey AS, Denny EG, Martin CW, O'Keefe J (2006) Phenology of a northern hardwood forest canopy.Global Change Biology, 12, 1174-1188. |
| 37 | Richardson AD, Braswell BH, Hollinger DY, Jenkins JP, Ollinger SV (2009) Near-surface remote sensing of spatial and temporal variation in canopy phenology.Ecological Applications, 19, 1417-1428. |
| 38 | Roy DB, Sparks TH (2000) Phenology of British butterflies and climate change.Global Change Biology, 6, 407-416. |
| 39 | Schwartz MD, Hanes JM (2010) Intercomparing multiple measures of the onset of spring in eastern North America.International Journal of Climatology, 30, 1614-1626. |
| 40 | Visser ME, Holleman LJM (2001) Warmer springs disrupt the synchrony of oak and winter moth phenology.Proceedings of the Royal Society of London Series B—Biological Sciences, 268, 289-294. |
| 41 | Wang HJ, Dai JH, Ge QS (2012) The spatiotemporal characteristics of spring phenophase changes of Fraxinus chinensis in China from 1952 to 2007.Science China-Earth Sciences, 55, 991-1000. |
| 42 | Willis CG, Ruhfel BR, Primack RB, Miller-Rushing AJ, Losos JB, Davis CC (2010) Favorable climate change response explains non-native species' success in Thoreau's woods.PLOS ONE, 5, e8878. |
| 43 | Wolkovich EM, Cook BI, Allen JM, Crimmins TM, Betancourt JL, Travers SE, Pau S, Regetz J, Davies TJ, Kraft NJB, Ault TR, Bolmgren K, Mazer SJ, McCabe GJ, McGill BJ, Parmesan C, Salamin N, Schwartz MD, Cleland EE (2012) Warming experiments underpredict plant phenological responses to climate change.Nature, 485, 494-497. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn ![]()