



生物多样性 ›› 2016, Vol. 24 ›› Issue (1): 95-101. DOI: 10.17520/biods.2015195 cstr: 32101.14.biods.2015195
所属专题: 传粉生物学
收稿日期:2015-07-06
接受日期:2015-09-15
出版日期:2016-01-20
发布日期:2016-06-12
通讯作者:
任明迅
基金资助:
Zhenna Qian1,2, Mingxun Ren1,2,*( )
)
Received:2015-07-06
Accepted:2015-09-15
Online:2016-01-20
Published:2016-06-12
Contact:
Ren Mingxun
摘要:
以金虎尾科植物地理分布格局及迁移历史总结出来的“金虎尾路线”, 是解释热带植物洲际间断分布与长距离扩散格局的重要模式。金虎尾路线阐明了金虎尾科植物历史时期7次独立的从起源中心(南美洲)向旧世界(非洲和亚洲)的洲际长距离扩散事件。本文总结了金虎尾路线植物起源地与扩散地主要类群的花部特征与传粉系统, 以探讨这些类群及类似植物长距离扩散后的花进化与传粉转变等适应规律。金虎尾科的南美洲类群都有分泌油脂的萼片腺体, 花的形态结构非常保守, 是与美洲当地特有的条蜂科集油蜂长期协同进化的结果。金虎尾科的非洲类群花保守性消失, 花白色、辐射对称且无萼片腺体, 繁育系统为雄花-两性花异株(功能性的雌雄异株), 传粉者是采集花粉的蜜蜂科昆虫。亚洲的一些属发生了类似非洲类群的泛化适应转变, 但风筝果属(Hiptage)出现了镜像花、异型雄蕊和极度反折的花瓣, 且传粉者是亚洲特有的大蜜蜂(Apis dorsata), 显示出了非常特化的适应性转变。风筝果属所在支系的现存类群涵盖了南美洲、中美洲、非洲和亚洲等地的地方特有属, 体现了金虎尾路线整个迁移历史过程, 是认识金虎尾路线及其进化适应规律的关键类群, 值得在今后的研究中加以重视。
钱贞娜, 任明迅 (2016) “金虎尾路线”植物的花进化与传粉转变. 生物多样性, 24, 95-101. DOI: 10.17520/biods.2015195.
Zhenna Qian, Mingxun Ren (2016) Floral evolution and pollination shifts of the “Malpighiaceae route” taxa, a classical model for biogeographical study. Biodiversity Science, 24, 95-101. DOI: 10.17520/biods.2015195.
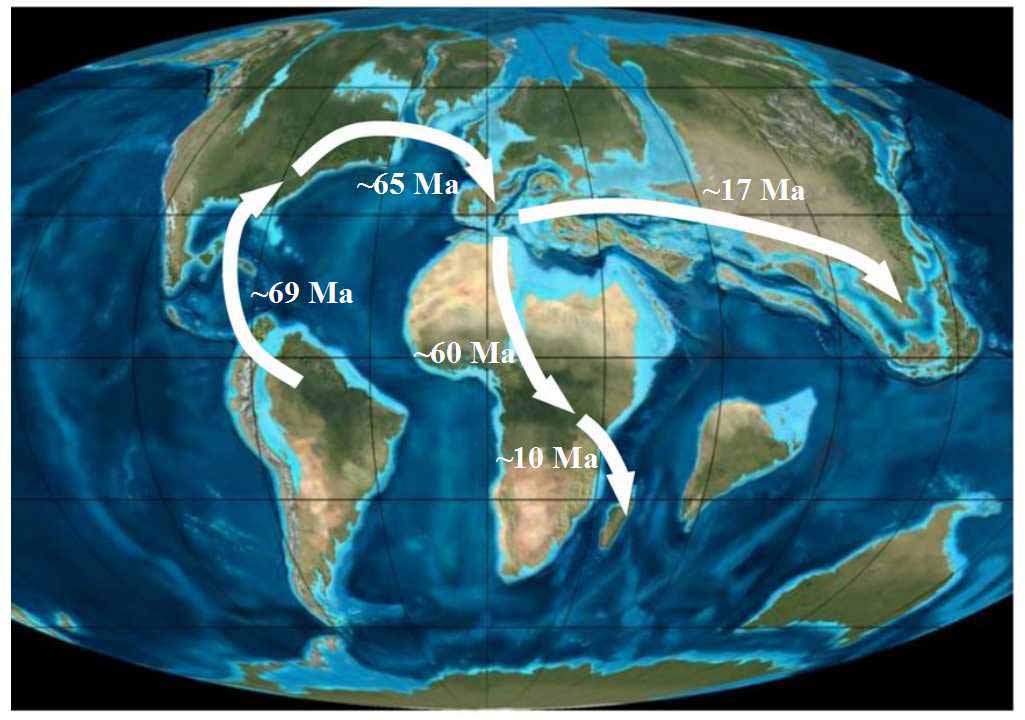
图1 金虎尾路线示意图。金虎尾科植物起源于大约75 Ma的南美洲北部, 之后逐渐迁移至北美洲, 再通过北大西洋陆桥扩散到劳亚古陆及后来的非洲与亚洲等地(背景地图为约65 Ma的始新世)。扩散路线旁的数字为大约的发生时间(根据Davis et al, 2010, 2014)。
Fig. 1 The sketch map of “Malpighiaceae route”. The family is proposed to be originated at about 75 Ma at the north of South America and its current distributions are proposed to have resulted by migration from South America to the Old World via land bridges in North Atlantic Ocean during Eocene (~ 65 Ma). The time near the dispersal route is adapted from Davis et al (2010, 2014).
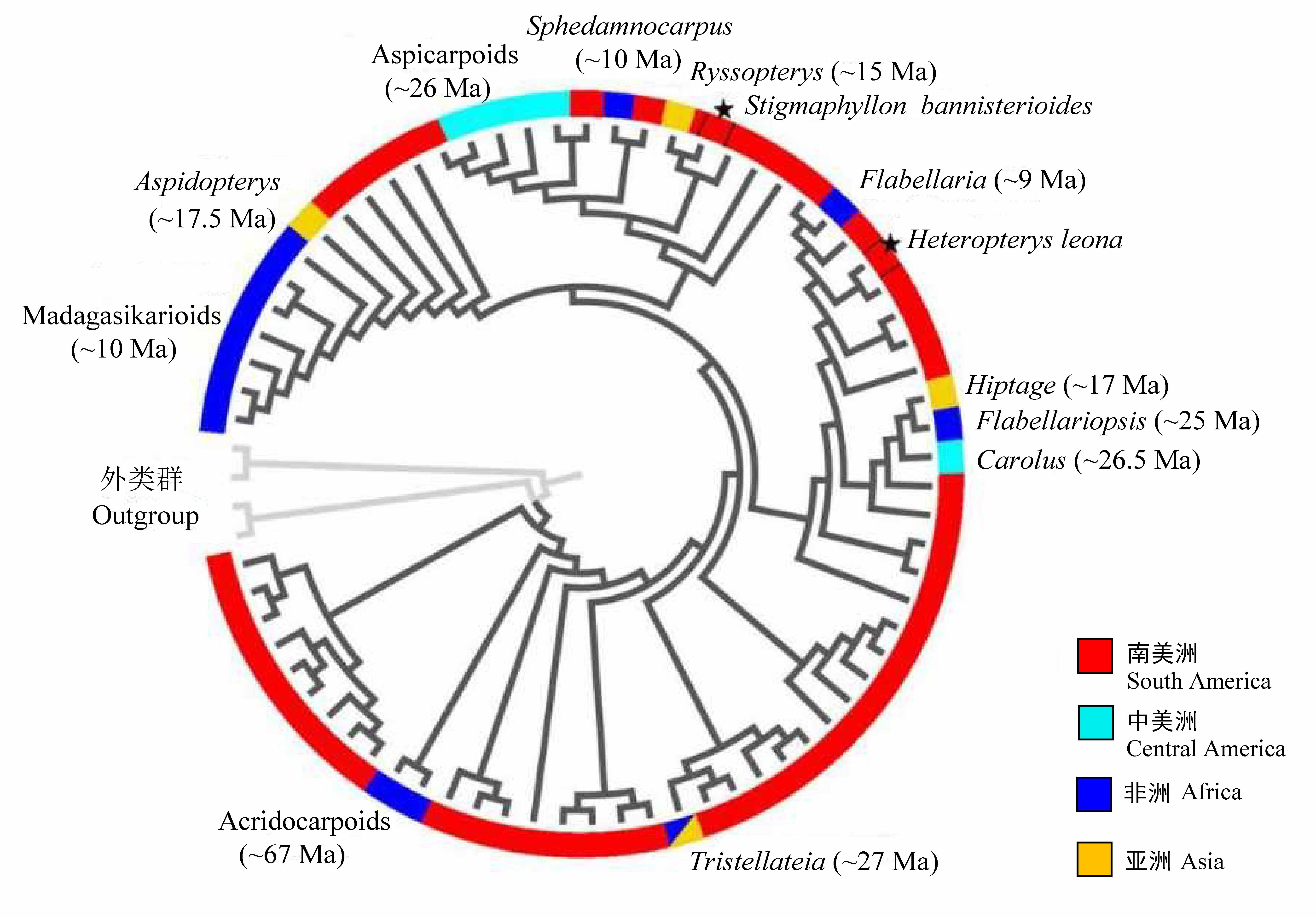
图2 “金虎尾路线”包括了7次历史时期的从美洲(南美洲、中美洲)到旧世界(非洲、亚洲)的长距离扩散事件。金虎尾科另有2次最近发生的美洲-非洲西海岸的直接横渡大西洋的迁移(★)。系统树及迁移历史时间根据Davis等(2014)。
Fig. 2 Malpighiaceae migrate independently into Old World (Africa and Asia) from South and Central America a total of seven times in the ancient time, with two very recent arrives to the west coast of Africa directly across the Atlantic Ocean (★). Phylogeny tree and the dispersal time are obtained from Davis et al (2014).
| 繁育系统 Breeding system | 花对称性 Floral symmetry | 花萼腺体 Calyx glands | 传粉者 Pollinators | 参考文献 References | |
|---|---|---|---|---|---|
| 南美洲 South America | 两性花 Hermaphrodite | 两侧对称 Bilateral symmetry | 10个油脂腺体 (每个萼片2个) 10 oil glands, paired on each sepal | 美洲特有的条蜂科集油蜂 America-endemic oil-collecting bees, including Centridini, Tetrapediini, and Tapinotaspidini | Anderson, 1979; Davis & Anderson, 2010; Davis et al, 2014 |
| 中美洲、北美洲 Central and North America | 两性花(闭花受精) Hermaphrodite (cleistogamy) | 辐射对称 Radial symmetry | 无 None | 花内自交, 不需传粉者 Autogamy (pollinator not needed) | Anderson, 1980 |
| 非洲 Africa | 雄花两性花异株(功能性雌雄异株) Androdioecy (functional dioecy) | 辐射对称 Radial symmetry | 无 None | 收集花粉的蜜蜂科昆虫 Pollen-gathering bees | Davis, 2002; Davis et al, 2014 |
| 亚洲 Asia | 两性花(镜像花) Hermaphrodite (mirror- image flowers) | 两侧对称 Bilateral symmetry | 无或1 (如有, 分泌糖) None or single (if present, secretes nectar) | 收集花粉的大蜜蜂(Apis dorsata) Asian giant bees (Apis dorsata) collecting pollen | Ren et al, 2013; Ren, 2015 |
表1 金虎尾科植物在不同大洲的主要花部特征、繁育系统及传粉者
Table 1 Breeding system, floral syndromes and pollinators of Malpighiaceae in different continents
| 繁育系统 Breeding system | 花对称性 Floral symmetry | 花萼腺体 Calyx glands | 传粉者 Pollinators | 参考文献 References | |
|---|---|---|---|---|---|
| 南美洲 South America | 两性花 Hermaphrodite | 两侧对称 Bilateral symmetry | 10个油脂腺体 (每个萼片2个) 10 oil glands, paired on each sepal | 美洲特有的条蜂科集油蜂 America-endemic oil-collecting bees, including Centridini, Tetrapediini, and Tapinotaspidini | Anderson, 1979; Davis & Anderson, 2010; Davis et al, 2014 |
| 中美洲、北美洲 Central and North America | 两性花(闭花受精) Hermaphrodite (cleistogamy) | 辐射对称 Radial symmetry | 无 None | 花内自交, 不需传粉者 Autogamy (pollinator not needed) | Anderson, 1980 |
| 非洲 Africa | 雄花两性花异株(功能性雌雄异株) Androdioecy (functional dioecy) | 辐射对称 Radial symmetry | 无 None | 收集花粉的蜜蜂科昆虫 Pollen-gathering bees | Davis, 2002; Davis et al, 2014 |
| 亚洲 Asia | 两性花(镜像花) Hermaphrodite (mirror- image flowers) | 两侧对称 Bilateral symmetry | 无或1 (如有, 分泌糖) None or single (if present, secretes nectar) | 收集花粉的大蜜蜂(Apis dorsata) Asian giant bees (Apis dorsata) collecting pollen | Ren et al, 2013; Ren, 2015 |
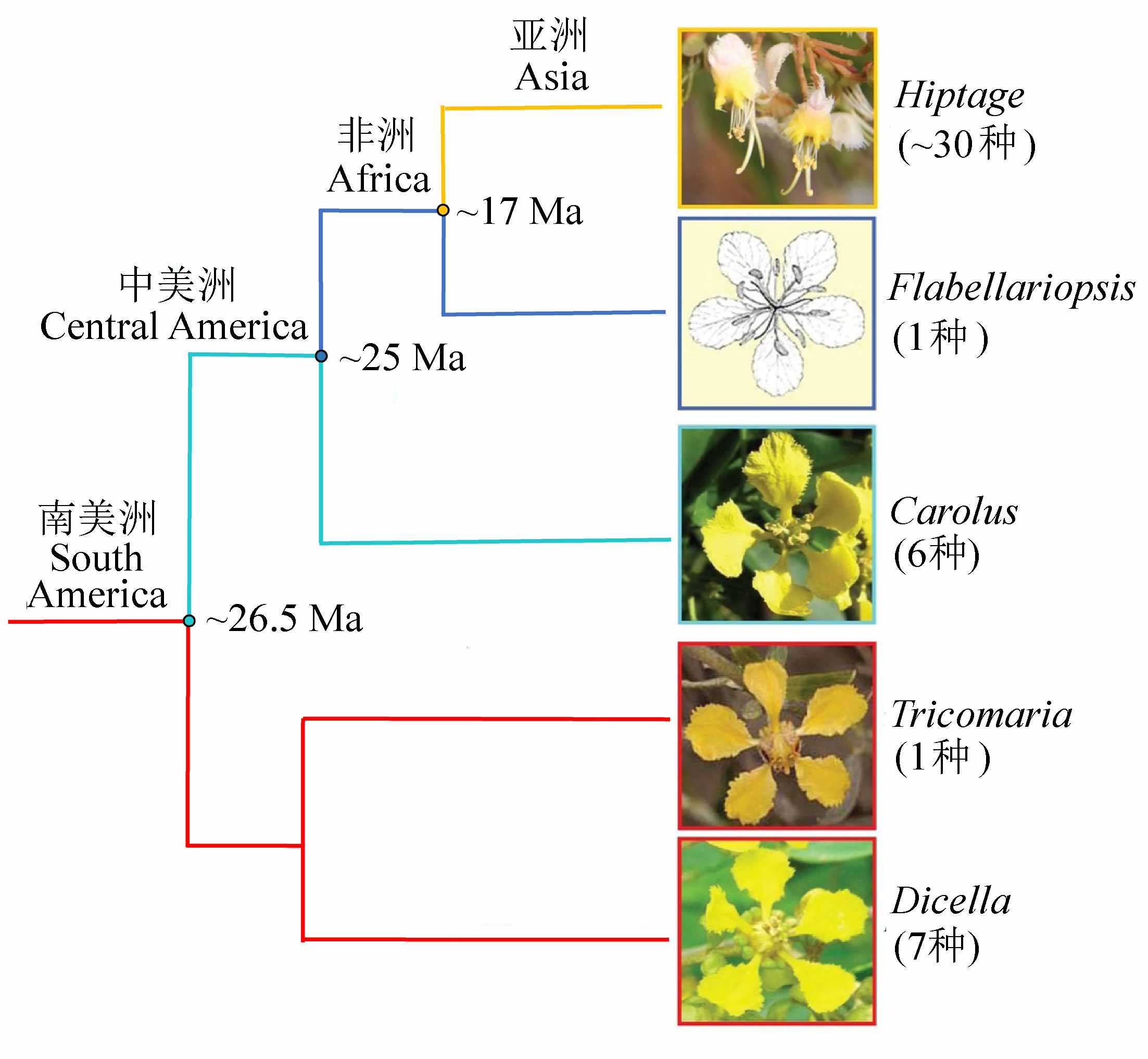
图3 亚洲特有的风筝果属(Hiptage)的分化与迁移历史是整个金虎尾路线扩散路径最完整、最具代表性的支系, 现存类群在南美洲、中美洲、非洲以及亚洲都有各自特有属(属名后为该属物种数)。系统树改自Davis和Anderson (2010)。
Fig. 3 The Asia-endemic Hiptage is in the clade with complete migration history of Malpighiaceae route, having endemic genera respectively in America, Africa, and Asia. The number in the brackets is the species diversity of the genus. Phylogeny relationships are determined according to Davis & Anderson (2010).
| 1 | Anderson WR (1979) Floral conservatism in neotropical Malpighiaceae. Biotropica, 11, 219-223. |
| 2 | Anderson WR (1980) Cryptic self-fertilization in the Malpighiaceae. Science, 207, 892-893. |
| 3 | Anderson WR (1981) Malpighiaceae in the botany of the Guayana Highland. Part XI. Memoirs of the New York Botanical Garden, 32, 45-48. |
| 4 | Anderson WR (1990) The origin of the Malpighiaceae: the evidence from morphology. Memoirs of the New York Botanical Garden, 64, 210-224. |
| 5 | Anderson WR, Anderson C, Davis CC (2006) Malpighiaceae.[accessed <date-in-citation content-type="access-date">2015-06-19</date-in-citation>] |
| 6 | Cappellari SC, Haleem MA, Marsaioli AJ, Tidon R, Simpson BB (2011) Pterandra pyroidea: a case of pollination shift within Neotropical Malpighiaceae. Annals of Botany, 107, 1323-1334. |
| 7 | Chanderbali AS, van der Werff H, Renner SS (2001) Phylogeny and historical biogeography of Lauraeeae: evidence from the chloroplast and nuclear genomes. Annals of Missouri Botanical Garden, 88, 104-134. |
| 8 | Chen SK, Funton AM (2008) Malpighiaceae. In: Flora of China, Volume 11 (Eds Wu ZY, Raven PH, Hong DY), pp. 135-138. Science Press, Beijing & Missouri Botanical Garden Press, St. Luis. |
| 9 | Davis CC (2002) Madagasikaria (Malpighiaceae): a new genus from Madagascar with implications for floral evolution in Malpighiaceae. American Journal of Botany, 89, 699-706. |
| 10 | Davis CC, Bell CD, Mathews S, Donoghue MJ (2002) Laurasian migration explains Gondwanan disjunctions: evidence from Malpighiaceae. Proceedings of the National Academy of Sciences, USA, 99, 6833-6837. |
| 11 | Davis CC, Anderson WR (2010) A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. American Journal of Botany, 97, 2031-2048. |
| 12 | Davis CC, Schaefer H, Xi ZX, Baum DA, Donoghue MJ, Harmon LJ (2014) Long-term morphological stasis maintained by a plant-pollinator mutualism. Proceedings of the National Academy of Sciences, USA, 111, 5914-5919. |
| 13 | Fritseh PW (2001) Phylogeny and biogeography of the flowering plant genus Styrax (Styraeaceae) based on chloroplast DNA restriction sites and DNA sequences of the internal transeribed spacer region. Molecular Phylogenetics and Evolution, 19, 387-408. |
| 14 | Gates B (1982) Banisteriopsis, Diplopterys (Malpighiaceae). Flora Neotropica, 30, 1-237. |
| 15 | Jesson LK, Barrett SCH (2002) Solving the puzzle of mirror-image flowers. Nature, 417, 707. |
| 16 | Jesson LK, Barrett SCH (2003) The comparative biology of mirror-image flowers. International Journal of Plant Sciences, 164, 237-249. |
| 17 | Lin Y, Tan DY (2007) Enantiostyly in angiosperms and its evolutionary significance. Acta phytotaxonomica Sinica, 45, 901-916. (in Chinese with English abstract) |
| [林玉, 谭敦炎(2007) 被子植物镜像花柱及其进化意义. 植物分类学报, 45, 901-916.] | |
| 18 | Ren MX (2015) The upper reaches of the largest river in Southern China as an ‘evolutionary front’ of tropical plants: evidences from Asia-endemic genus Hiptage (Malpighiaceae). Collectanea Botanica, 34, e002. |
| 19 | Ren MX, Zhang DY (2004) Herkogamy. In: Plant Life-History Evolution and Reproductive Ecology (ed. Zhang DY), pp. 303-320. Science Press, Beijing. (in Chinese) |
| [任明迅, 张大勇 (2004) 雌雄异位. 见: 植物生活史进化与繁殖生态学 (张大勇主编). 科学出版社, 北京.] | |
| 20 | Ren MX, Zhong YF, Song XQ (2013) Mirror-image flowers without buzz pollination in the Asia-endemic Hiptage benghalensis (Malpighiaceae). Botanical Journal of the Linnean Society, 173, 764-774. |
| 21 | Renner SS, Clausing G, Meyer K (2001) Historical biogeography of Melastomataceae: the roles of tertiary migration and long-distance dispersal. American Journal of Botany, 88, 1290-1300. |
| 22 | Renner SS, Schaefer H (2010) The evolution and loss of oil-offering flowers: new insights from dated phylogenies for angiosperms and bees. Philosophical Transactions of the Royal Society of London, Series B, 365, 423-435. |
| 23 | Steiner KE (1985) Functional dioecism in the Malpighiaceae: The breeding system of Spachea membranacea Cuatr. American Journal of Botany, 72, 1537-1543. |
| 24 | Vogel S (1990) History of the Malpighiaceae in the light of pollination ecology. Memoirs of the New York Botanical Garden, 55, 130-142. |
| 25 | Week A, Daly AW, Simpson BB (2005) The phylogenetic history and biogeography of the frankincense and myrrh family (Burseraeeae) based on nuclear and chloroplast sequence data. Molecular Phylogenetics and Evolution, 35, 85-101. |
| 26 | Zhang WH, Kramer EM, Davis CC (2010) Floral symmetry genes and the origin and maintenance of zygomorphy in a plant-pollinator mutualism. Proceedings of the National Academy of Sciences, USA, 107, 6388-6393. |
| 27 | Zhou ZK, Yang XF, Yang QS (2006) Land bridge and long-distance dispersal—old ideas, new evidence. Chinese Science Bulletin, 51, 897-886. (in Chinese) |
| [周浙昆, 杨雪飞, 杨青松 (2006) 陆桥说和长距离扩散——老观点, 新证据. 科学通报, 51, 879-886.] |
| [1] | 丁翔, 余元钧, 宋希强, 罗毅波. 具有泛化访花者的海芋特化传粉系统[J]. 生物多样性, 2024, 32(6): 24069-. |
| [2] | 孙思邈, 陈吉欣, 冯炜炜, 张昶, 黄凯, 管铭, 孙建坤, 刘明超, 冯玉龙. 植物氮形态利用策略及对外来植物入侵性的影响[J]. 生物多样性, 2021, 29(1): 72-80. |
| [3] | 孙士国, 卢斌, 卢新民, 黄双全. 入侵植物的繁殖策略以及对本土植物繁殖的影响[J]. 生物多样性, 2018, 26(5): 457-467. |
| [4] | 黄至欢, 陆奇丰, 陈颖卓. 地锦苗在石灰岩土壤和红壤生境中的繁殖成功的比较[J]. 生物多样性, 2017, 25(9): 972-980. |
| [5] | 钱贞娜, 孟千万, 任明迅. 风筝果镜像花的雌雄异位变化及传粉生态型的形成[J]. 生物多样性, 2016, 24(12): 1364-1372. |
| [6] | 钟云芳, 张哲, 宋希强, 周兆德. 海南凤仙花不同海拔种群的传粉生物学[J]. 生物多样性, 2014, 22(4): 467-475. |
| [7] | 叶建飞, 陈之端, 刘冰, 覃海宁, 杨永. 中国西南与台湾地区维管植物的间断分布格局及形成机制[J]. 生物多样性, 2012, 20(4): 482-494. |
| [8] | 刘欣欣, 吴小琴, 张奠湘. 艳丽耳草的二型花柱及异型自交不亲和系统[J]. 生物多样性, 2012, 20(3): 337-347. |
| [9] | 路国辉, 武文华, 王瑞珍, 李新亮, 王英强. 野牡丹异型雄蕊的功能分化[J]. 生物多样性, 2009, 17(2): 174-181. |
| [10] | 贾效成, 李新亮, 丹阳, 路国辉, 王英强. 广东地区外来种五爪金龙的传粉生物学研究[J]. 生物多样性, 2007, 15(6): 592-598. |
| [11] | 张敬丽, 张长芹, 吴之坤, 乔琴. 探讨种间传粉在杜鹃花属自然杂交物种形成中的作用[J]. 生物多样性, 2007, 15(6): 658-665. |
| [12] | 王仲礼, 刘林德, 田国伟, 申家恒. 短柄五加开花及传粉生物学研究*[J]. 生物多样性, 1997, 05(4): 251-256. |
| [13] | 王洪新, 胡志昂. 植物的繁育系统、遗传结构和遗传多样性保护[J]. 生物多样性, 1996, 04(2): 92-96. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn