



生物多样性 ›› 2011, Vol. 19 ›› Issue (3): 311-318. DOI: 10.3724/SP.J.1003.2011.08239 cstr: 32101.14.SP.J.1003.2011.08239
所属专题: 物种形成与系统进化
收稿日期:2010-11-01
接受日期:2011-02-05
出版日期:2011-05-20
发布日期:2013-12-10
通讯作者:
葛学军,曹同
作者简介:ct1946@263.net基金资助:
Yan Liu1, Jianxiu Wang2, Xuejun Ge3,*( ), Tong Cao4,*(
), Tong Cao4,*( )
)
Received:2010-11-01
Accepted:2011-02-05
Online:2011-05-20
Published:2013-12-10
Contact:
Xuejun Ge,Tong Cao
摘要:
对于苔藓植物DNA条形码研究来说, 目前已提议的可用片段只有rbcL和trnH-psbA, 并且均具有一定局限性。本文基于GenBank中3,365条rps4序列, 利用遗传距离法和分子系统学方法评价它作为苔藓植物候选条形码的可行性。结果显示: (1)rps4序列覆盖了藓纲96%的科和苔纲88%的科, 具有通用性; (2)rps4物种分辨能力为73.0%, 并且它在6个序列最丰富的苔藓植物属(Plagiochila, Tortula, Plagiomnium, Pyrrhobryum, Pogonatum, Grimmia)内的物种识别能力均高于rbcL-a在同属中的分辨能力; (3)GenBank中已经积累了大量已知物种来源的rps4序列, 可为DNA条形码物种鉴定提供一个参考数据库。因此, 本文建议将rps4作为苔藓植物候选DNA条形码。尤其是当rbcL和trnH-psbA在某个具体类群中无法取得理想的物种识别效果时, rps4可作为补充。
刘艳, 王建秀, 葛学军, 曹同 (2011) rps4作为苔藓植物候选条形码的可行性: 基于GenBank数据的分析. 生物多样性, 19, 311-318. DOI: 10.3724/SP.J.1003.2011.08239.
Yan Liu, Jianxiu Wang, Xuejun Ge, Tong Cao (2011) The rps4 locus as an alternative marker for barcoding bryophytes: eva- luation based on data mining from GenBank. Biodiversity Science, 19, 311-318. DOI: 10.3724/SP.J.1003.2011.08239.
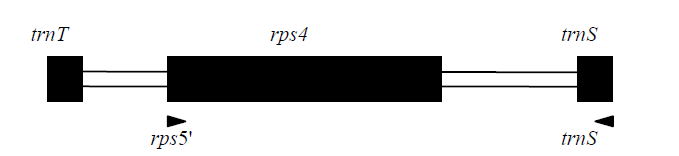
图1 rps4通用引物的位置和方向。黑色长方形框代表编码区, 白色长方形框代表非编码区。
Fig. 1 The positions and directions of the primers used to amplify the rps4 cpDNA locus. Coding regions are highlighted by black boxes, whereas the non-coding regions are highlighted by white boxes.
| 种数 No. of species | 属数 No. of genera | 科数 No. of families | 目数 No. of orders | 序列数目 No. of accessions | |
|---|---|---|---|---|---|
| 藓纲 Bryophyta | 1,580 | 692 | 108 | 30 | 2,604 |
| 苔纲 Marchantiophyta | 498 | 184 | 74 | 15 | 756 |
| 角苔纲 Anthocerotophyta | 4 | 3 | 2 | 2 | 5 |
| 总计 Total | 2,082 | 879 | 184 | 47 | 3,365 |
表1 GenBank中苔藓植物rps4序列特点
Table 1 Characteristics of rps4 sequences from bryophytes available in GenBank
| 种数 No. of species | 属数 No. of genera | 科数 No. of families | 目数 No. of orders | 序列数目 No. of accessions | |
|---|---|---|---|---|---|
| 藓纲 Bryophyta | 1,580 | 692 | 108 | 30 | 2,604 |
| 苔纲 Marchantiophyta | 498 | 184 | 74 | 15 | 756 |
| 角苔纲 Anthocerotophyta | 4 | 3 | 2 | 2 | 5 |
| 总计 Total | 2,082 | 879 | 184 | 47 | 3,365 |
| 种内、种间遗传距离 数值的数目 Total no. of pair-wise comparisons | 物种识别率 Species resolution (%) | 无法识别的物种比例 Species unresolved (%) | |||
|---|---|---|---|---|---|
| 无种间变异 No inter-specific divergence (%) | 种内距离≥种间距离 Intra-specific distance ≥ inter-specific distance (%) | 种内变异> 0.2%* Intra-specific variations > 0.2%* (%) | |||
| 藓纲 Bryophyta | 19,664 | 70.7 (876/1,239) | 17.8 (221/1,239) | 7.6 (94/1,239) | 3.9 (48/1,239) |
| 苔纲 Marchantiophyta | 12,896 | 79.9 (333/417) | 9.6 (40/417) | 8.4 (35/417) | 2.2 (9/417) |
| 总计 Total | 3,2560 | 73.0 (1,209/1,656) | 15.8 (261/1,656) | 7.8 (129/1,656) | 3.4 (57/1,656) |
表2 基于K2P遗传距离分析rps4位点的物种识别能力
Table 2 Species resolution by the rps4 locus based on K2P distances
| 种内、种间遗传距离 数值的数目 Total no. of pair-wise comparisons | 物种识别率 Species resolution (%) | 无法识别的物种比例 Species unresolved (%) | |||
|---|---|---|---|---|---|
| 无种间变异 No inter-specific divergence (%) | 种内距离≥种间距离 Intra-specific distance ≥ inter-specific distance (%) | 种内变异> 0.2%* Intra-specific variations > 0.2%* (%) | |||
| 藓纲 Bryophyta | 19,664 | 70.7 (876/1,239) | 17.8 (221/1,239) | 7.6 (94/1,239) | 3.9 (48/1,239) |
| 苔纲 Marchantiophyta | 12,896 | 79.9 (333/417) | 9.6 (40/417) | 8.4 (35/417) | 2.2 (9/417) |
| 总计 Total | 3,2560 | 73.0 (1,209/1,656) | 15.8 (261/1,656) | 7.8 (129/1,656) | 3.4 (57/1,656) |
| 种数 No. of species | 序列数 No. of accessions | 物种识别率 Species resolution (%) | 种间无变异 No inter-specific divergence (%) | 种内距离≥种间距离 Intra-specific distance ≥ inter-specific distance (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rps4 | rbcL-a | rps4 | rbcL-a | rps4 | rbcL-a | rps4 | rbcL-a | rps4 | rbcL-a | |||||
| 羽苔属 Plagiochila | 128 | 111 | 147 | 125 | 82 | 77.5 | 8.6 | 20.7 | 9.4 | 1.8 | ||||
| 墙藓属 Tortula | 18 | 4 | 97 | 5 | 61.1 | - | 11.1 | - | 27.8 | - | ||||
| 匍灯藓属 Plagiomnium | 24 | 5 | 87 | 17 | 66.7 | 40 | 0 | 60 | 33.3 | 0 | ||||
| 桧藓属 Pyrrhobryum | 10 | 9 | 55 | 16 | 60 | 44.4 | 20 | 44.4 | 20 | 11.1 | ||||
| 小金发藓属 Pogonatum | 32 | 18 | 50 | 32 | 68.8 | 55.6 | 21.9 | 11.1 | 9.4 | 33.3 | ||||
| 紫萼藓属 Grimmia* | 35 | 29 | 49 | 71 | 71.4 | 58.6 | 22.9 | 20.7 | 5.7 | 20.7 | ||||
表3 rps4与rbcL-a在6个苔藓植物属内物种识别能力的比较
Table 3 Comparison of species resolution between rps4 and rbcL-a in six bryophyte genera
| 种数 No. of species | 序列数 No. of accessions | 物种识别率 Species resolution (%) | 种间无变异 No inter-specific divergence (%) | 种内距离≥种间距离 Intra-specific distance ≥ inter-specific distance (%) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rps4 | rbcL-a | rps4 | rbcL-a | rps4 | rbcL-a | rps4 | rbcL-a | rps4 | rbcL-a | |||||
| 羽苔属 Plagiochila | 128 | 111 | 147 | 125 | 82 | 77.5 | 8.6 | 20.7 | 9.4 | 1.8 | ||||
| 墙藓属 Tortula | 18 | 4 | 97 | 5 | 61.1 | - | 11.1 | - | 27.8 | - | ||||
| 匍灯藓属 Plagiomnium | 24 | 5 | 87 | 17 | 66.7 | 40 | 0 | 60 | 33.3 | 0 | ||||
| 桧藓属 Pyrrhobryum | 10 | 9 | 55 | 16 | 60 | 44.4 | 20 | 44.4 | 20 | 11.1 | ||||
| 小金发藓属 Pogonatum | 32 | 18 | 50 | 32 | 68.8 | 55.6 | 21.9 | 11.1 | 9.4 | 33.3 | ||||
| 紫萼藓属 Grimmia* | 35 | 29 | 49 | 71 | 71.4 | 58.6 | 22.9 | 20.7 | 5.7 | 20.7 | ||||
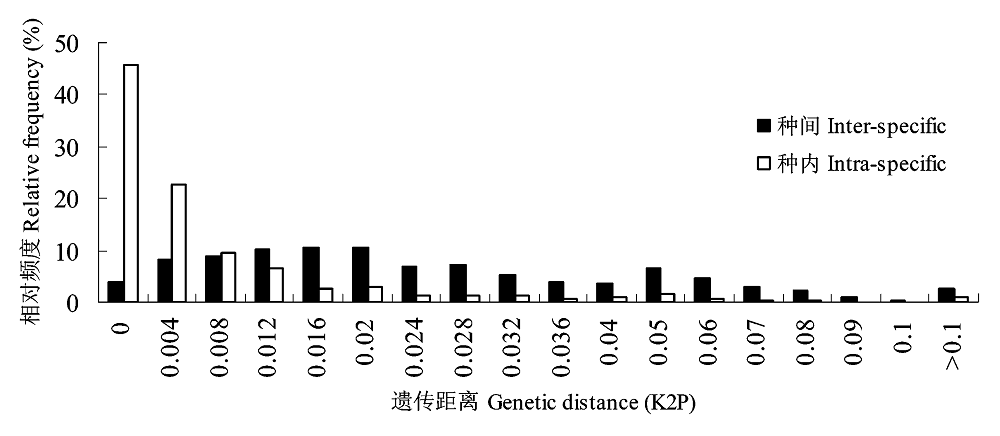
图2 基于GenBank中rps4序列的种内、种间K2P遗传距离分布图
Fig. 2 Frequency histograms of intra- and inter-specific pair-wise distances (K2P) based on the rps4 region data available from GenBank
| [1] | CBOL Plant Working Group (2009) A DNA barcode for land plants. Proceedings of the National Academy of Sciences, USA, 106, 12794-12797. |
| [2] | Chase MW, Cowan RS, Hollingsworth PM, van den Berg C, Madriñán S, Petersen G, Seberg O, Jørgsensen T, Cameron KM, Carine M, Pedersen N, Hedderson TAJ, Conrad F, Salazar GA, Richardson JE, Hollingsworth ML, Barraclough TG, Kelly L, Wilkinson M (2007) A proposal for a standardised protocol to barcode all land plants. Taxon, 56, 295-299. |
| [3] |
Chase MW, Salamin N, Wilkinson M, Dunwell JM, Kesanakurthi RP, Haidar N, Savolainen V (2005) Land plants and DNA barcodes: short-term and long-term goals. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences, 360, 1889-1895.
URL PMID |
| [4] |
Der JP, Thomson JA, Stratford JK, Wolf PG (2009) Global chloroplast phylogeny and biogeography of bracken (Pteridium; Dennstaedtiaceae). American Journal of Botany, 96, 1041-1049.
URL PMID |
| [5] | Devey DS, Chase MW, Clarkson JJ (2009) A stuttering start to plant DNA barcoding: microsatellites present a previously overlooked problem in non-coding plastid regions. Taxon, 58, 7-15. |
| [6] | Edwards D, Horn A, Taylor D, Savolainen V, Hawkins JA (2008) DNA barcoding of a large genus,Aspalathus L.(Fabaceae). Taxon, 57, 1317-1327. |
| [7] | Fahleson J, Okori P, Åkerblom-Espeby L, Dixelius C (2008) Genetic variability and genomic divergence of Elymus repens and related species. Plant Systematics and Evolution, 271, 143-156. |
| [8] |
Fazekas AJ, Burgess KS, Kesanakurti PR, Graham SW, Newmaster SG, Husband BC, Percy DM, Hajibabaei M, Barrett SCH (2008) Multiple multilocus DNA barcodes from the plastid genome discriminate plant species equally well. PLoS ONE, 3, e2802. doi: 10.1371/journal.pone.0002802.
DOI URL PMID |
| [9] | Fazekas AJ, Kesanakurti PR, Burgess KS, Percy DM, Graham SW, Barrett SCH, Newmaster SG, Hajibabaei M, Husband BC (2009) Are plant species inherently harder to discriminate than animal species using DNA barcoding markers? Molecular Ecology Resources, 9(s1), 130-139. |
| [10] | Gernandt DS, López GG, García SO, Liston A (2005) Phylogeny and classification of Pinus. Taxon, 54, 29-42. |
| [11] |
Goffinet B, Cox CJ, Shaw AJ, Hedderson TAJ (2001) The bryophyta (mosses): systematic and evolutionary inferences from an rps4 gene (cpDNA) phylogeny. Annals of Botany, 87, 191-208.
DOI URL PMID |
| [12] | Goffinet B, Shaw AJ (2009) Bryophyte Biology, 2nd edn. Cambridge University Press, Cambridge. |
| [13] |
Hajibabaei M, Singer GAC, Hebert PDN, Hickey DA (2007) DNA barcoding: how it complements taxonomy, molecular phylogenetics and population genetics. Trends in Genetics, 23, 167-172.
URL PMID |
| [14] |
Hebert PDN, Cywinska A, Ball SL, deWaard JR (2003) Biological identifications through DNA barcodes. Proceedings of the Royal Society of London Series B: Biological Sciences, 270, 313-321.
DOI URL PMID |
| [15] |
Hollingsworth ML, Clark AA, Forrest LL, Richardson J, Pennington RT, Long DG, Cowan R, Chase MW, Gaudeul M, Hollingsworth PM (2009) Selecting barcoding loci for plants: evaluation of seven candidate loci with species-level sampling in three divergent groups of land plants. Molecular Ecology Resources, 9, 439-457.
URL PMID |
| [16] | Kelly LJ, Ameka GK, Chase MW (2010) DNA barcoding of African Podostemaceae (river-weeds): a test of proposed barcode regions. Taxon, 59, 251-260. |
| [17] |
Kress WJ, Erickson DL (2007) A two-locus global DNA barcode for land plants: the coding rbcL gene complements the non-coding trnH-psbA spacer region. PLoS ONE, 2, e508. doi: 10.1371/journal.pone.0000508.
DOI URL PMID |
| [18] | Kress WJ, Wurdack KJ, Zimmer EA, Weigt LA, Janzen DH (2005) Use of DNA barcodes to identify flowering plants. Proceedings of the National Academy of Sciences, USA, 102, 8369-8374. |
| [19] | Lahaye R, van der Bank M, Bogarin D, Warner J, Pupulin F, Gigot G, Maurin O, Duthoit S, Barraclough TG, Savolainen V (2008) DNA barcoding the floras of biodiversity hotspots. Proceedings of the National Academy of Sciences, USA, 105, 2923-2928. |
| [20] |
Li CX, Lu SG (2006) Phylogenetics of Chinese Dryopteris (Dryopteridaceae) based on the chloroplast rps4-trnS sequence data. Journal of Plant Research, 119, 589-598.
DOI URL PMID |
| [21] | Liu Y, Cao T, Ge XJ (2011) A case study of DNA barcoding in Chinese Grimmiaceae and a moss recorded in China for the first time. Taxon, 60, 185-193. |
| [22] |
Liu Y, Yan HF, Cao T, Ge XJ (2010) Evaluation of 10 plant barcodes in Bryophyta (Mosses). Journal of Systematics and Evolution, 48, 36-46.
DOI URL |
| [23] | Nadot S, Bajon R, Lejeune B (1994) The chloroplast gene rps4 as a tool for the study of Poaceae phylogeny. Plant Systematics and Evolution, 191, 27-38. |
| [24] | Newmaster SG, Fazekas AJ, Ragupathy S (2006) DNA barcoding in the land plants: evaluation of rbcL in a multigene tiered approach. Canadian Journal of Botany, 84, 335-341. |
| [25] | Newmaster SG, Ragupathy S (2009) Testing plant barcoding in a sister species complex of pantropical Acacia (Mimosoideae, Fabaceae). Molecular Ecology Resources, 9(s1), 172-180. |
| [26] |
Pennisi E (2007) Wanted: a barcode for plants. Science, 318, 190-191.
DOI URL PMID |
| [27] | Ragupathy S, Newmaster SG, Murugesan M, Balasubramaniam V (2009) DNA barcoding discriminates a new cryptic grass species revealed in an ethnobotany study by the hill tribes of the Western Ghats in southern India. Molecular Ecology Resources, 9(s1), 164-171. |
| [28] |
Seberg O, Petersen G (2009) How many loci does it take to DNA barcode a Crocus? PLoS ONE, 4, e4598. doi: 10.1371/journal.pone.0004598.
DOI URL PMID |
| [29] | Shaw AJ (2001) Biogeographic patterns and cryptic speciation in bryophytes. Journal of Biogeography, 28, 253-261. |
| [30] | Souza-Chies TT, Bittar G, Nadot S, Carter L, Besin E, Lejeune B (1997) Phylogenetic analysis of Iridaceae with parsimony and distance methods using the plastid gene rps4. Plant Systematics and Evolution, 204, 109-123. |
| [31] |
Steele PR, Friar LM, Gilbert LE, Jansen RK (2010) Molecular systematics of the neotropical genus Psiguria (Cucurbitaceae): implications for phylogeny and species identification. American Journal of Botany, 97, 156-173.
URL PMID |
| [32] |
Stenøien HK (2008) Slow molecular evolution in 18S rDNA,rbcL and nad5 genes of mosses compared with higher plants. Journal of Evolutionary Biology, 21, 566-571.
DOI URL PMID |
| [33] | Swofford DL (2003) PAUP*: Phylogenetic Analysis Using Parsimony (*and Other Methods). Version 4.0 b10. Sinauer Associates, Sunderland. |
| [34] |
Taberlet P, Coissac E, Pompanon F, Gielly L, Miquel C, Valentini A, Vermat T, Corthier G, Brochmann C, Willerslev E (2007) Power and limitations of the chloroplast trnL (UAA) intron for plant DNA barcoding. Nucleic Acids Research, 35, e14.
URL PMID |
| [35] |
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Molecular Biology and Evolution, 24, 1596-1599.
URL PMID |
| [36] |
Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Research, 25, 4876-4882.
DOI URL PMID |
| [37] | Vitt DH, Wieder RK (2009) The structure and function of bryophyte-dominated peatlands. In: Bryophyte Biology, 2nd edn. (eds Goffinet B, Shaw AJ), pp. 357-392. Cambridge University Press, Cambridge. |
| [38] |
Werner O, Guerra J (2004) Molecular phylogeography of the moss Tortula muralis Hedw. (Pottiaceae) based on chloroplast rps4 gene sequence data. Plant Biology, 6, 147-157.
DOI URL PMID |
| [39] | Werner O, Ros RM, Cano MJ, Guerra J (2004) Molecular phylogeny of Pottiaceae (Musci) based on chloroplast rps4 sequence data. Plant Systematics and Evolution, 243, 147-164. |
| [40] | Wyatt R, Odrzykoski IJ (1998) On the origins of the allopolyploid moss Plagiomnium cuspidatum. Bryologist, 101, 263-271. |
| [41] | Wyatt R, Odrzykoski IJ, Stoneburner A (1992) Isozyme evidence of reticulate evolution in mosses:Plagiomnium medium is an allopolyploid of P. ellipticum × P. insigne. Systematic Botany, 17, 532-550. |
| [42] | Wyatt R, Odrzykoski IJ, Stoneburner A (1993) Isozyme evidence regarding the origins of the allopolyploid moss Plagiomnium curvatulum. Lindbergia, 18, 49-58. |
| [43] | Wyatt R, Odrzykoski IJ, Stoneburner A, Bass HW, Galau GA (1988) Allopolyploidy in bryophytes: multiple origins of Plagiomnium medium. Proceedings of the National Academy of Sciences, USA, 85, 5601-5604. |
| [44] | Zander RH (1993) Genera of Pottiaceae: Mosses of Harsh Environments. Buffalo Society of Natural Sciences, Buffalo. |
| [1] | 徐聪, 张飞宇, 俞道远, 孙新, 张峰. 土壤动物的分子分类预测策略评估[J]. 生物多样性, 2022, 30(12): 22252-. |
| [2] | 全东丽, 杨斌, 马文章, 宋亮, 沈婷. 西双版纳苔藓植物多样性及其濒危状况[J]. 生物多样性, 2021, 29(4): 545-553. |
| [3] | 曹云, 沈文静, 陈炼, 胡飞龙, 周蕾, 徐海根. Metabarcoding技术在真菌多样性研究中的应用[J]. 生物多样性, 2016, 24(8): 932-939. |
| [4] | 王太, 张艳萍, 管丽红, 杜岩岩, 娄忠玉, 焦文龙. 甘肃省鱼类资源现状及DNA条形码在鱼类物种鉴定中的应用[J]. 生物多样性, 2015, 23(3): 306-313. |
| [5] | 张珰妮, 郑连明, 何劲儒, 张文静, 林元烧, 李阳. 基于线粒体COI和16S片段序列的北部湾北部水螅水母DNA条形码分析[J]. 生物多样性, 2015, 23(1): 50-60. |
| [6] | 顾海峰, 刘婷婷, 蓝东兆. 中国沿海甲藻包囊研究进展[J]. 生物多样性, 2011, 19(6): 779-786. |
| [7] | 李超伦, 王敏晓, 程方平, 孙松. DNA条形码及其在海洋浮游动物生态学研究中的应用[J]. 生物多样性, 2011, 19(6): 805-814. |
| [8] | 隆沂峄, 杨丽媛, 廖万金. 利用PCR-RFLP技术鉴定传粉榕小蜂隐种混合样品的物种组成[J]. 生物多样性, 2010, 18(4): 414-419. |
| [9] | 李春香, 陆树刚. 龙骨星蕨与膜叶星蕨的关系:来自叶绿体rbcL、rps4和trnL-trnF、rps4-trnS序列的证据[J]. 生物多样性, 2005, 13(2): 174-179. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2026 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
![]()