花是植物的生殖器官, 分为单性花和两性花。单性花是指只包含雄蕊或雌蕊的花, 两性花是指在一朵花中同时包含雄蕊和雌蕊。一株植物同时具有两种性别的单性花称为雌雄同株(monoecious), 具有两性花的植株称为雌雄同花(hermaphrodite), 只具有单一性别的单性花植株称为雌雄异株(dioecious)。不同类别的植物中, 雌雄异株物种所占的比例不尽相同。苔藓植物谱系中, 68%的苔藓、57%的地钱和40%的角苔植物为雌雄异株(Villarreal & Renner, 2013); 种子植物内存在一个相对较大的差异现象, 即裸子植物已报道的1,033个物种中, 667种都是雌雄异株(Walas et al, 2018); 而在被子植物中雌雄异株较为罕见, 只占所有被子植物物种的6% (Renner & Ricklefs, 1995)。相比动物而言, 植物的性别分化方式更为复杂。除了雌雄同株、雌雄异株物种外, 还存在雌/雄全同株、雌/雄全异株物种, 即雌雄同株和一种性别分离个体同时存在于一个个体或自然群体中, 以及雌性、雄性个体和两性个体同时存在于一个自然群体的现象(Westergaard, 1958; Spigler et al, 2008)。
雌雄异株物种性别决定的相关研究一直是植物学家研究的热点课题, 根据已有的研究成果不难发现, 被子植物雌雄异株物种广泛分布在不同的进化谱系之中(表1)。已报道的大多数物种为XY型性别系统, 少数为与鸟类相同的ZW型, 这两种不同的性别系统可能存在于同一个科或属的物种中, 如杨柳科(Yang et al, 2021)。另外也存在极少数物种为XO型性别决定系统(Charlesworth, 2019)。随着近年来测序成本的降低和分析技术水平的提高, 利用基因组学的方法, 已经有多个物种的性别决定区域和基因得到鉴定, 其中被子植物物种包括较早期的蝇子草(Silene latifolia) (Sondur et al, 1996; Delph et al, 2010; Kazama et al, 2016; Lorenzo et al, 2018)、番木瓜(Carica papaya) (Liu et al, 2004; Wang et al, 2012; Aryal & Ming, 2014)和近几年的芦笋(Asparagus officinalis) (Harkess et al, 2017, 2020)、猕猴桃(Actinidia chinensis) (Akagi et al, 2018, 2019)、杨树(Müller et al, 2020; Xue et al, 2020; Yang et al, 2021)、大麻(Cannabis sativa) (Prentout et al, 2020)等。尽管发现的大多数性别决定基因在相似的激素反应途径中发挥作用, 但基因本身并不相同, 性染色体所处的进化状态差异也非常大, 这种现象说明雌雄异株进化的过程有着许多相互独立的途径(Ming et al, 2011)。
表1 典型代表植物性别决定的研究成果
Table 1
| 类群 Taxon | 物种 Species | 性别决定系统 Sex determination system | 性别决定区域或基因 Sex-linked region or genes | 同源基因或家族 Ortholog gene or family | 参考文献 Reference |
|---|---|---|---|---|---|
| 苔藓植物 Bryophyte | 地钱 Marchantia polymorpha | XY | 14个雄性特异性基因 14 male-specific genes | - | Yamato et al, 2007 |
| 裸子植物 Gymnosperm | 银杏 Ginkgo biloba | ZW | GbMADS18, Gb_15883, Gb_15884, Gb_15885, Gb_15886, Gb_28587 | MADS-box (GbMADS18), RR12 (Gb_15883), RR2 (Gb_15884), ELF6 (Gb_15885), AtBAT1 (Gb_15886), AGL8 (Gb_28587) | Zhang et al, 2019; Liao et al, 2020 |
| 被子植物 Angiosperm | 番木瓜 Carica papaya | XY | MSY4-5Mb, HSY8.1 Mb, XSY3.5 Mb | - | Liu et al, 2004; Wang et al, 2012 |
| 菠菜 Spinacia oleracea | XY | 4号连锁群 LG4 (66.98-69.72 cM and 75.48-92.96 cM) | - | Qian et al, 2017; She et al, 2021 | |
| 杨梅 Myrica rubra | ZW | 59 kb 8号染色体雌性特异性片段 59 kb female-specific region on chromosome 8 | - | Jia et al, 2019 | |
| 大麻 Cannabis sativa | XY | 性染色体 Sex chromosomes | - | Prentout et al, 2020 | |
| 蝇子草 Silene latifolia | XY | SlAP3, SlSTM, SlCUC | AP3 (SlAP3), STM (SlSTM), CUC1/CUC2 (SlCUC) | Zluvova et al, 2006; Cegan et al, 2010 | |
| 草莓 Fragaria virginiana | ZW | GMEW, RPP0W | GDP-mannose 3,5-epimerase 2 (GMEW), 60S acidic ribosomal protein P0 (RPP0W) | Charlesworth, 2013; Tennessen et al, 2018 | |
| 葡萄 Vitis vinifera | XY | VviINP1, VviYABBY3 | INP1 (VviINP1), YAB1 (VviYABBY3) | Massonnet et al, 2020 | |
| 君迁子 Diospyros lotus | XY | MeGI, OGI | HB40 (MeGI) | Akagi et al, 2020; Xue et al, 2020 | |
| 芦笋 Asparagus officinalis | XY | SOFF, aspTDF1 | DUF247 (SOFF), TDF1 (aspTDF1) | Harkess et al, 2017, 2020 | |
| 猕猴桃 Actinidia chinensis | XY | SyGl, FrBy | ARR24 (SyGl), FAS1 (FrBy) | Akagi et al, 2018, 2019 | |
| 杨树 Populus deltoides, P. tremula, P. alba | XY, ZW | FERR-R, FERR, MmS, ARR17 | ARR17 | Müller et al, 2020; Xue et al, 2020 | |
| 柳树 Salix purpurea, S. triandra | ZW | RR | RR9/ARR17 | Li et al, 2020; Zhou et al, 2020 |
现结合国内外具有代表性和阶段性意义的性别决定研究成果, 从植物性别决定基因模型、植物性染色体进化过程、性别决定区域和基因、性别决定的研究方法以及性别决定研究的实际应用和前景进行综述。
1 双基因和单基因决定模型
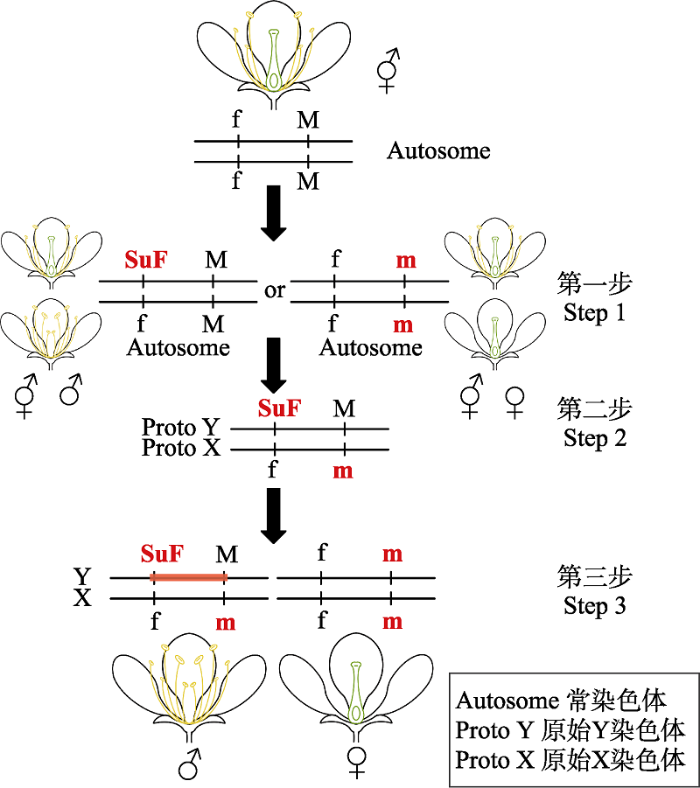
开花植物的雌雄异株通常被认为是从雌雄同株或雌雄同花进化而来(Charlesworth, 2016)。一个物种由两性系统转变为性别分离的物种至少需要两个基因突变: 一个突变影响胚珠的产生, 另一个突变则影响花粉的产生(Westergaard, 1958)。Brian Charlesworth和Deborah Charlesworth曾提出一个双基因突变模型假说, 即从常染色体进化到有效的Y染色体至少涉及到两个基因: 一个是抑雌因子(the suppressor of feminization, SuF)的显性突变, 另一个是导致雄性不育的隐性突变(Charlesworth, 2015) (图1); 并推测在进化过程中, 首先产生雄性不育突变并在群体里传播, 产生分离的雌性个体, 然后出现雌性不育突变, 产生最终的雌雄异株物种(Charlesworth & Charlesworth, 1978)。这种双基因决定性别的设想下, SuF的获得需要具有显性效应的功能增益突变, 以便在杂合子(例如XY异形配子)的雄性中能发挥效应, 后续的许多关于雌雄异株植物的研究都支持植物性别决定的双基因模型。如Harkess等(2017)在园林芦笋性别决定文章中通过基因组组装鉴别出两个性别决定基因: 一个是潜在的促雄因子(M) aspTDF1, 另一个是潜在的抑雌因子(SuF) SOFF。Akagi等(2018, 2019)在猕猴桃的性别决定研究中发现了一对基因Shy Girl (SyGl)、Friendly Boy (FrBy), 分别具有促使心皮不育和促进花药生长的功能。
图1
图1
双基因性别决定模型(改编自Charlesworth (2015))。Step 1: 物种某对常染色体其中一条获得某种性别不育基因突变; Step 2: 另一条染色体获得对应另一种性别不育基因的突变, 形成性染色体前体; Step 3: 不育基因形成连锁, 性染色体初步形成。其中f→SuF为显性突变, M→m为隐性突变, Step 3中Y染色体阴影部分为不育基因形成连锁。
Fig. 1
Sex determination genes of ‘two-mutations’ model (adapted from Charlesworth (2015)). Step 1: One of a pair of autochromosomes has acquired a mutation of a certain sex-sterile gene; Step 2: The other one autochromosome gains the mutation corresponding to another sex sterile gene, forming the precursor of sex chromosome; Step 3: Sterility genes are linked and sex chromosomes are preliminarily formed. ‘f’ to ‘SuF’ is a dominant mutation, and ‘M’ to ‘m’ is a recessive mutation. The shaded part of Y chromosome in Step 3 represents the linkage of sterile genes.
促进雄性或抑制雌性的性别突变会显著降低雌性相关功能的适合度, 即促雄基因和促雌基因之间存在遗传冲突(genetic conflict) (Werren & Beukeboom, 1998), 从而促使多个雄性增益基因连锁遗传。但如果促雄或抑雌突变的产生不降低雌性的适合度, 并且在雄性个体中足够有利, 这种突变即使不与雌性相关基因发生连锁, 也可以在群体中传播, 产生单基因性别决定系统(Charlesworth, 2015)。单基因性别决定系统的植物如二倍体君迁子(Diospyros lotus), 性别决定由OGI控制, 当其存在时植株发育为雄性, 不存在的则发育为雌性(Akagi et al, 2014)。另外, 在已经形成的性别决定系统中, 新出现的性别突变基因可能取代原有的性别决定基因, 形成新的单基因决定系统(Charlesworth, 2012)。在被子植物雌雄异株进化的众多起源假设中, 单基因和双基因的性别决定模型之间并不冲突。
2 植物性染色体的进化
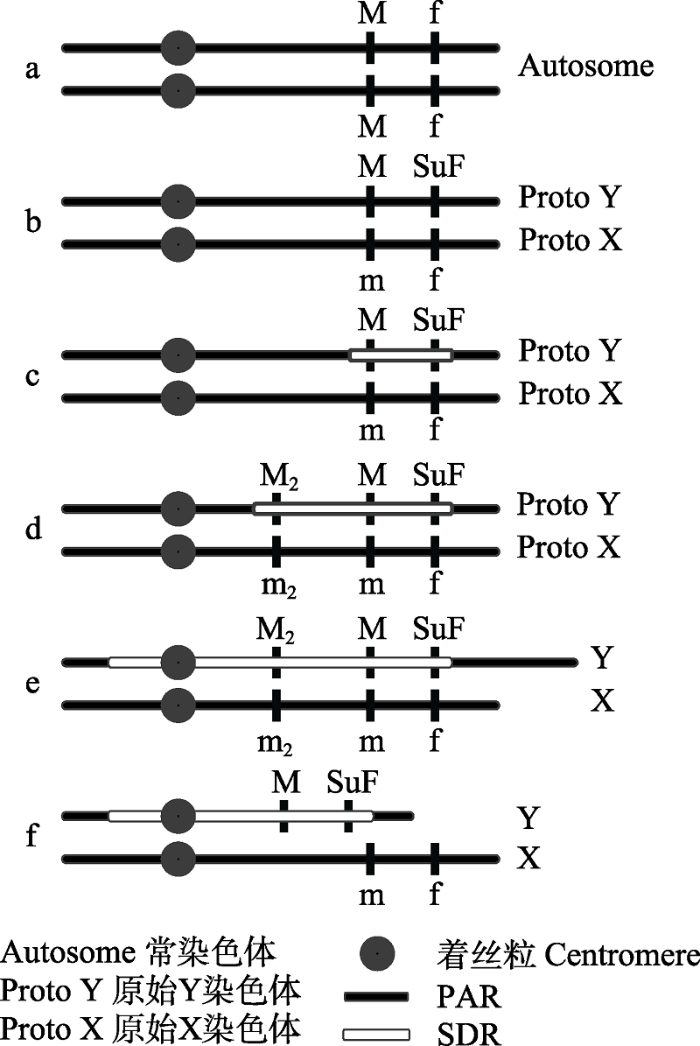
图2
图2
性染色体进化推论(改编自Ming et al (2011))。a, 常染色体; b, 两个性别决定基因形成; c, 性别决定基因连锁, 形成初步性别决定区域; d, 更多性别相关基因连锁, 性别决定区域扩大; e, 由于重复序列累积, Y染色体增长; f, Y染色体退化; PAR, 拟常染色体区域; SDR, 性别决定区域; f→SuF为显性突变, M→m为隐性突变; M2→m2为雄性功能基因的隐性突变。
Fig. 2
Sex chromosome evolution corollary (adapted from Ming et al (2011)). a, Autosomes; b, Two sex-determining genes are formed; c, Sex determination genes are linked, forming a preliminary sex determination region; d, More sex related genes are linked, and the sex-determined region expands; e, The Y chromosome increases due to the accumulation of repeated sequences; f, Y chromosome degeneration; PAR, Pseudoautosomal regions; SDR, Sex-determined area. ‘f’ to ‘SuF’ is a dominant mutation, and ‘M’ to ‘m’ is a recessive mutation. ‘M2’ to ‘m2’ is a recessive mutation of another male functional gene.
由于两性存在不同的生殖策略, 它们对于不同性状产生的选择压力也不同甚至相反, 一些在一种性别中有益的等位基因在另一种性别中可能是有害的(Werren & Beukeboom, 1998), 在遗传冲突的影响下逐渐累积这些性拮抗突变基因, 这个过程促进重组抑制(recombination suppression)的形成(Charlesworth et al, 2005)。随后, 性别决定基因之间产生连锁, 重组抑制由性别决定基因位点逐渐往性拮抗突变基因方向扩散, 形成早期染色体的性别决定区域(sex determined region, SDR) (图2c), 重组抑制是两条性染色体开始发生遗传分化的必要条件(Charlesworth et al, 2005)。此时尚未有明确的X (Z)、Y (W)染色体之分。以芦笋为例, 该物种的XY染色体在细胞学上是同形的, 存在XX、XY、YY三种染色体类型的个体。Harkess等(2017)对芦笋的研究发现其Y染色体存在一段约1 Mb的非重组区域, 且YY个体中的13个雄性特异性基因中的12个位于该非重组区域, 这些基因在雌性个体中(XX)未能鉴别出来。
随着越来越多的对维持一种性别有利的基因在性别决定基因附近被选择固定, 性别决定区域逐渐扩大, 出现重复元件的积累和基因组片段的复制或易位, 从而重组抑制被进一步加强, 导致性拮抗基因和性别决定基因座之间完全连锁(图2d)。由于重组抑制引起的希尔罗伯森效应(Hill-Robertson effect)使得Y染色体逐渐退化, 丢失部分X染色体上的基因逐渐形成性别特异性区域, 这个过程可能导致YY个体致死(Ming et al, 2011)。此阶段的XY染色体间虽然存在染色体结构上的差异, 但在细胞学上无法被检测, 依旧是同型的。2004年Liu等人关于番木瓜的性别研究发现, 番木瓜的性别受到3种等位基因控制: M雄性、Mh两性和m雌性, 但MM、MMh以及MhMh致死, 对其性别特异性区域的重复元件进行分析发现, 雄性特异性区域(male-specific region)比常染色体区域的反向元件密度高27.6%, 比反向重复序列密度高188.9% (Liu et al, 2004), 说明在番木瓜的性染色体中, 雄性特异性区域与对应染色体区域的重组受到严重抑制。
重复序列的不断积累使得Y染色体的重组抑制逐渐扩展到染色体的大多数区域, 致使XY染色体异形, 在结构、功能和基因密度上出现较大差异(图2e)。与哺乳动物较为短小的Y染色体不同, 开花植物性别分化出现较晚, Y染色体积累的大量重复序列和转座子导致其形态比常染色体还要长。如对叉枝蝇子草(Silene latifolia)的性别相关研究发现, 其XY染色体在约10 Mya出现分化, 两条染色体大小相差很大, 其中Y染色体长度约为X染色体的1.4倍。Y染色体上的非重组区域占整条染色体的绝大部分, 拟常染色体区域(pseudoautosomal regions, PAR)的长度仅占其总长的10%。两条染色体都累积了各种转座子和卫星DNA, 并在组蛋白修饰和DNA甲基化方面存在明显的区别(Bačovský et al, 2020)。
3 性别决定区域和基因
3.1 性别决定区域研究
从雄性或者雌性材料中鉴定出每种植物中的性别特异性区域是研究性别决定的起点和关键步骤, 从已发表的文章来看, 该策略也是目前常用的有效策略之一。
番木瓜是研究性别决定的模式植物, 也是最早期研究植物性别的物种之一。从20世纪40年代开始到60年代, 关于番木瓜性别决定的重要理论成果被相继提出, 由Storey (1953)先后发现修正, 并最终确认番木瓜性别决定为经典的XX-XY型, 雄性、雌性与两性的基因型分别为XY、XX和XYh, 其中Y染色体上有个致死区域, Yh为Y染色体的突变体, 且木瓜的性别决定受性染色体上狭窄范围内一段序列中的多个基因控制(Ming et al, 2007)。随着遗传图谱和分子标记等技术运用于性别决定研究, Sondur等(1996)利用RAPD分子标记技术建立了一个由11个连锁群组成, 总长为999.3 cM的分子遗传连锁图谱, 其中将性别决定基因定位于两个遗传距离为14 cM的标记之间。Liu等(2004)利用AFLP分子标记技术建立了一个含有1,501个标记的高密度分子遗传连锁图谱, 结合BAC文库和SCAR标记技术估计出番木瓜雄性特异性区域(male-specific region of Y chromosome, MSY)长度大约为4-5 Mb, 且证实了该区域拥有高重复、非重组、快速进化的特征。Wang等(2012)通过BAC克隆与组装技术组装了番木瓜Yh两性染色体的特异性区域(hermaphrodite-specific region of Yh chromosome, HSY)和对应的X染色体特异性区域, 分别得到8.1 Mb和3.5 Mb的两段序列, 其中HSY序列由于反转录转座子插入, 长度比X染色体对应序列更长, 且存在两个大规模的倒位现象。番木瓜Y染色体特异性区域仅占染色体长度的10%左右, 是一个处于进化早期阶段的性染色体类型, 对番木瓜性染色体的研究可以探究染色体进化早期可能发生的事件, 为植物性别研究提供了非常丰富的理论和实践价值。
除番木瓜之外, 其他多种植物性别特异性区域的研究成果也先后被发表。菠菜(Spinacia oleracea)是一种广泛种植的叶菜类蔬菜, 早期的研究鉴定出其为XY性别决定系统, 雄性为XY, 雌性为XX (Janick & Stevenson, 1954)。Qian等(2017)基于SLAF-SEQ技术构建出菠菜的高密度遗传图谱, 并将性别决定基因定位于LG4 (66.98-69.72 cM和75.48-92.96 cM)处。随后She等(2021)根据10份重测序数据(雄性5株, 雌性5株), 在4号染色体上鉴定出一个约21 kb的雄性特异性区域(MSY)。同时开发出一个KASP标记SponR, 能够成功鉴定出菠菜不同基因型的植株。杨梅(Myrica rubra)是重要的水果类经济作物, Jia等(2018)采用混合二代和三代测序组装技术分别组装出雌性和雄性的基因组, 分别结合100个雌株和100个雄株混池测序以及3个雌株和3个雄株重测序, 检测到一段约为59 kb的雌性特异性区域(female-specific region, FSR), 并在FSR区域筛选到了杨梅性别决定候选基因。芦笋的性别决定位点M被定位在5号染色体上一段0.25 cM的区域中 (Harkess et al, 2017)。野生型葡萄(Vitis vinifera)的性别决定区域在2006年被定位到2号染色体上(Riaz et al, 2006)。随后Picq等(2014)精确到2号染色体一段154.8 kb的区域内。Prentout等(2020)利用RNA测序技术鉴定出超过500个性别连锁基因并将其比对至大麻基因组, 鉴别出大麻性染色体, 并在X染色体上鉴定出一段特异性序列, 根据性别连锁基因分析得出大麻的Y染色体已经严重退化, 可能为目前已知的最古老的植物性染色体系统。
3.2 性别决定基因研究
随着测序技术和分析手段不断优化, 以及前人研究成果的铺垫, 越来越多的植物性别研究逐渐深入到筛选和鉴别具体的性别决定基因上。Takashi Akagi课题组在植物性别决定研究上取得了许多成果。2014年该组研究人员结合转录组和进化的方法在君迁子中发现了一个Y特异性性别决定候选基因OGI (Akagi et al, 2014), 该基因在柿属(Diospyros)中比较保守, 编码一个可特异性剪切常染色体上MeGI基因的小RNA。在OGI存在的情况下, 编码的小RNA靶向MeGI抑制其表达, 抑制雌花发育, 促进雄花发育; 而雌花中不存在OGI基因时, MeGI正常表达, 抑制雄花发育, 促进雌花发育。后对六倍体君迁子的研究发现, 在OGI启动子区域插入一个长268 bp的Kali转座子元件可导致甲基化程度发生变化, 从而影响OGI的功能(Akagi et al, 2016)。2020年研究人员完成了一个完整君迁子基因组的组装, 通过进化分析得知该物种与猕猴桃属(Actinidia)分化后发生了一次谱系特异性的全基因组复制事件, 并提供了Y染色体的详细结构信息。同时鉴定出一个新的MeGI姐妹基因SiMeGI, 确定了3个基因MeGI、OGI和SiMeGI的旁系同源关系。进化分析还表明, MeGI基因在全基因组复制事件后经历了一次适应性进化突变, MeGI特异性地获得了一种抑制雄性器官发育的新功能, 而SiMeGI则可能保持了原有的功能。随后一个局部复制事件在Y染色体上产生了MeGI的调节因子OGI, 完成了雌雄异株的进化, 可能也由此导致Y染色体的形成(Akagi et al, 2020)。该课题组另一个研究物种猕猴桃的性别决定基因也得到鉴别, 2018年研究人员结合基因组测序和转录组分析鉴定到一个C类细胞分裂素响应调控子(type-C cytokinin response regulator), 作为猕猴桃潜在的一个性别决定基因, 在拟南芥和烟草中的功能转基因分析显示该基因是心皮发育的显性抑制子, 命名为SyGl (Akagi et al, 2018)。他们对几个猕猴桃属物种的进化分析表明, SyGl定位于基因组Y染色体特异的区域, 可能起源于支系特异性的基因复制事件, 其同源基因Achn384741与SyGl编码功能相同的蛋白, 但表达部位为嫩叶, 与SyGl完全不同。可能由于转录组取样时间过早导致花发育时期涵盖不完全, 此次研究并未发现促雄基因。2019年该课题组再次鉴定到了Y染色体上编码的另外一个控制猕猴桃性别决定的促雄基因FrBy, 且独立于SyGl基因。相关的重测序研究发现由于Y染色体上SyGl基因的缺失, 一个含有FrBy基因的群体均为雌雄同花个体。同时, 在雌性猕猴桃中表达FrBy基因也能够获得雌雄同花的猕猴桃个体(Akagi et al, 2019)。该课题组的两种不同研究结果均显示性别决定基因由基因复制事件产生, 说明这个过程可能是植物性别决定基因进化的重要途径, 并且恰好与早期提出的单基因与双基因模型吻合。
2017年Harkess等人完成了YY芦笋染色体的组装, 并找到了Y特异性区域, 完成了对SOFF基因和aspTDF1基因的寻找和鉴别(Harkess et al, 2017), 但aspTDF1基因的功能没有在所有雌雄异株的芦笋上起作用。2020年该课题组进行了XX的姊妹个体基因组组装, 鉴别到一段163 kb的X特异性区域(Harkess et al, 2020), 该区域两端序列与Y特异性区域序列相似, 并通过共线性分析排除了移位倒位现象。研究发现SOFF和aspTDF1没有在XX个体或是X同源区域中表达, 根据之前的研究推测aspTDF1可能是一个性别决定的分子基础, 处于进化阶段。后对SOFF和aspTDF1进行功能验证, 发现确实是两个性别决定基因, 推测aspTDF1可能不是唯一的雄性性别决定基因, 或许存在多个促雄性别决定基因的起源。Müller等(2020)对两类性别决定区域位置有差异的杨属(Populus)植物使用组装Y染色体和重测序鉴定出ARR17基因, 该基因发生不同位置的反向重复从而通过RNA介导DNA甲基化的过程决定杨树性别。随后, Yang等(2021)结合以往多个杨柳科的研究成果, 发现杨柳科植物性染色体上都存在编码A型细胞分裂素响应调控因子(type-A cytokinin response regulator, RR)的同源序列, 并提出了杨柳科植物性别系统频繁转换的分子遗传学普适模型, 即通过RR反向重复片段形成siRNA, 介导RR完整基因的甲基化修饰和转录沉默, 从而控制其发展形成XY性别决定系统; 而RR反向重复片段的丢失或去功能化, 则导致形成ZW性别决定系统。关于葡萄的研究, Massonnet等(2020)通过对8个葡萄品种进行组装和测序, 解析了20个葡萄的性别决定区域(SDR)单倍型, 明确了葡萄的SDR边界, 并将其分成了F、M和H 3种单倍型, 同时鉴别出VviINP1基因和转录因子VviYABBY3分别作为雄性和雌性不育的候选基因。
通过这些物种具体性别决定基因的研究不难发现, 虽然这些基因最终表型大多为雄蕊或心皮不育, 但其发育的基因和停滞时期各不相同, 这些雌雄异株植物所在的系统发育位置也较为分散, 染色体发育阶段也大不相同, 说明植物的性别进化在不同的植物谱系中独立演化, 发生的机制也存在差异。同时也进一步说明后续需要在更多的植物类群中进行更详实的性别决定研究。
4 性别决定的研究方法
随着性别决定研究的深入, 解析性别决定的方法也越来越多。特别是测序成本、技术和方法的快速发展, 使得人们可以更快更准地了解到植物性别决定的区域、机理和成因。目前, 可以应用到该领域的技术方法主要包括分子标记技术以及测序组装技术。
4.1 分子标记技术
早期的性别决定研究手段主要为各种DNA分子标记技术。作为一类基础的分子生物学技术, DNA分子标记技术主要包括: 随机扩增多态性DNA (random amplified polymorphism DNA, RAPD)标记、序列特异性扩增区(sequence-characterized amplified region, SCAR)标记、扩增片段长度多态性(amplified fragment length polymorphism, AFLP)标记、酶切扩增多态性序列(cleaved amplified polymorphism sequences, CAPS)标记和限制性片段长度多态性(restriction fragment length polymorphism, RFLP)标记等。
RAPD是早期最广泛应用于植物性别相关研究的标记手段之一, 基于DNA指纹差异, 利用PCR技术, 可以进行多态位点筛选, 寻找和识别性别特异性标记从而鉴定植物性别。该标记技术拥有操作简单、实验设备要求低、花费少等优点, 但由于实验中需要经历复杂的引物筛选过程, 且RAPD标记对实验条件比较敏感, 同时实验结果难重复, 缺乏可靠性。SCAR标记是在RAPD标记的基础上发展起来的, 在反应敏感度、重复性上较RAPD标记表现更好, 现在的研究分析通常利用引物将RAPD标记转化为更稳定的SCAR标记, 以获得更多的信息位点。目前雌雄异株植物性别连锁的RAPD标记和转化后的SCAR标记在许多物种, 如番木瓜、大麻、猕猴桃、芦笋等的早期性别决定中均有应用报道。RFLP标记是一种杂交分子标记技术, 是最早期发展的DNA分子标记之一, 利用探针杂交检测不同个体基因组中限制性内切酶酶切位点的突变, 从而比较不同个体DNA水平上的差异, 应用于遗传图谱的构建和基因定位等研究。但该技术操作繁琐、花费较高, 应用受到极大限制。AFLP标记技术是基于RAPD和RFLP技术发展而来, 结合二者高重复性和操作简便的优点, 避免了制备探针、敏感性差等缺点, 该技术能够同时检测大量的遗传位点和多态性标记, 广泛应用于植物性别鉴定的研究中。
早期分子标记为性别决定研究开通了一条全新的道路, 但分子标记技术仅能得到各标记基因之间的距离, 还需结合其他研究手段才能粗略估计染色体大小和性别决定区域长短。总体来说, 早期分子标记技术缺乏一定的精度, 无法对上下游基因和整体代谢通路进行研究。
4.2 基因组、转录组测序和组装
细菌人工染色体(bacterial artificial chromosome, BAC)克隆组装技术是早期研究性别特异性区域相对精确的手段, 该技术的发明是基因组研究中一个较大且重要的转折点, 由此研究人员对性别决定的探索由性别决定区域逐渐转向关键基因的挖掘。
随着测序技术的发展, 基因组相关的二代、三代测序和组装技术逐渐应用于研究, 越来越多的植物物种性别决定相关机制得到解析。猕猴桃是经典的利用二代测序和组装技术研究植物性别决定的物种(Akagi et al, 2018, 2019)。研究人员结合二代基因组和转录组测序, 将基因组和转录组测序数据(reads, 150 bp)分别打断成更小的30 bp和35 bp的Kmer片段, 筛选含有雄性个体特有Kmer片段的reads进行组装。随后将组装好的重叠群(contigs)比对到已有的雌性猕猴桃参考基因组上, 通过相似性将contigs分为Y染色体特异重叠群(Y-specific contigs)、Y染色体等位基因完全连锁重叠群(Y-allelic fully sex-linked contigs)和Y染色体等位基因部分连锁重叠群(Y-allelic fully sex-linked contigs), 同时将Y特异性contigs进一步组装成一段约为0.49 Mb的MSY序列。结合基因组、转录组contigs基因结构注释和差异表达分析筛选出2条雄性特异性contigs的61个基因, 进一步根据表达部位和个体差异现象确定了1个编码C型细胞分裂素反应调节因子的候选基因, 命名为SyGl。后续功能验证证实了该基因为猕猴桃性别决定基因中的抑雌基因。该课题组另外的研究也通过转录组数据用相同方法发现猕猴桃的促雄基因FrBy, 并利用Y染色体组装注释和基因表达进一步佐证SyGl和FrBy是猕猴桃的两个性别决定基因。二代测序的低成本、高通量和高准确性的特性使植物性别决定的研究速度有了质的提升, 但受限于固定且较短的插入片段长度, 使得测序数据在重复序列方面的组装效果稍有欠缺。三代测序则弥补了二代读长短的缺陷, 测序reads长度可以跨越大部分重复区域, 在重复序列部分可以组装得更加完整, 有利于后续对重复序列部分的分析。XY性别系统和ZW系统在杨柳科内反复转换的模型被证明与RR基因复制插入染色体的不同位置有关, 而杨属内RR基因的插入则可能由Helitron转座子和类Copia转座子片段的插入捕获部分RR基因, 从而驱动RR基因的复制和插入(Yang et al, 2021), 而这都得益于三代长片段测序技术的发展和组装。
重测序技术测序和全基因组关联分析也是鉴别定位植物性别决定基因的方法之一, 通过对群体在全基因组范围内的遗传变异位点与表型进行关联分析, 筛选与性状显著相关的变异位点从而定位基因。美洲黑杨(Populus deltoides)的性别相关研究中, 研究人员利用49个雌株和46个雄株进行重测序, 以雌性P. deltoides个体和其F2代雄性个体组装的两个基因组为参考基因组, 分别进行全基因组关联分析, 定位到TCP、CLC、MET1、FERR、FERR-R和MmS这几个基因中, 后结合转录组等技术进一步确定FERR-R是抑雌基因, 产生siRNAs抑制FERR的雌性功能, 而MmS则能够促进雄性功能表达(Müller et al, 2020)。由此可见, 测序技术和方法的发展正在加速植物性别决定的解析步伐, 会有越来越多具有不同性别系统的机制被解析出来。
5 性别决定研究的实际应用和前景
雌雄异株植物由于性别差异, 其群体性别比例与经济价值和应用方向有着直接的关系。以果实种子为主要经济来源的植物, 提高其雌性植株占比可以大大增加产量以提升经济价值, 如猕猴桃、黄瓜等瓜果蔬菜, 需通过提高雌株(雌花)的比例从而提高产量; 而主要收获营养器官, 或是用于各种行道树、景观绿化的植物使用更多的是雄性个体, 这种类型的物种应提高雄性个体比例从而获得更高的经济价值, 如杨树、柳树等, 通过筛选雄树减少杨絮柳絮的产生。而许多植物幼生期较长, 在开花前的营养期雌雄株差异并不十分明显, 若通过花器官鉴别植物性别则存在耗时长、效率低等问题, 所以在幼生期鉴定雌雄异株植物性别则显得尤为重要。
目前在生产实践中已有许多方法可以进行植株雌雄性别鉴定。部分植物不同性别的个体在外表上存在一定的差异, 通过形态差异鉴别植株性别是最为方便快捷的方法之一。陶应时等(2013)对连香树雌雄株叶片形态的比较研究发现, 雄株的叶长、叶宽、叶片表面积、叶片的鲜质量和干质量等指标均显著高于雌株叶片。部分植物性别不同, 其酶活力、次生代谢物和内源激素水平方面存在较为明显的差异。李国梁等(1993)利用HPLC色谱图分别对杨梅、猕猴桃和银杏(Ginkgo biloba)进行水溶性酚类含量测定, 发现银杏雌株的咖啡酸和某种未知酚类的相对含量比雄株高; 杨梅和猕猴桃的水溶性酚类含量存在显著差异, 雌株酚类含量显著高于雄株, 且在猕猴桃中表现更为明显, 并利用水溶性酚类总量将大多数实生苗鉴定出性别。王白坡等(1999)发现银杏芽尖及叶片中的GA3、ZT、IAA和ABA在雌株和雄株间均有较为明显的差异。但生理生化指标容易受到各种客观条件的影响, 例如环境、取样时间、取样部位等, 准确性容易受到干扰。傅力等(2019)根据同种植物雌雄株叶片中化学成分不同引起的电化学行为差异鉴定部分植物的性别。还有多种其他方法, 例如同工酶图谱鉴别、染色体组鉴别、化学药剂处理鉴别等已投入实际应用中。
随着研究越来越深入, 鉴别植物性别的方法逐渐从外形、蛋白质、激素等方向转向染色体和DNA序列层面。王白坡等(1999)发现银杏雌雄株除了在内源激素方面存在差异外, 核酸含量在不同性别的个体中也不同。Hormaza等(1994)运用RAPD标记技术在阿月浑子(Pistacia vera)中找到一段945 bp雌性特异性区域标记。She等(2021)在菠菜中从与MSR紧密连锁的SNP (T/A)中开发了一个KASP标记SponR, 并对958个个体(475个雌株, 483个雄株)进行了检测, 成功将XX、XY和YY 3种基因型区分开来。但由于不同植物种类间存在较大差异, 许多雌雄异株物种幼生期在外形上无区别, 差异代谢物检测困难或其他方面的原因, 致使目前的植物性别鉴定方法不具有普适性, 许多未开展研究或是研究基础比较薄弱的物种依旧无法区分植株性别。而基因组学的发展将可以加速植物雌雄特异序列的开发, 进而研发快速有效的分子标记进行不同性别个体的精准鉴定。
6 展望
性别决定被称为“达尔文之谜”, 一直以来是植物进化研究的重点方向。雌雄异株在植物谱系中拥有很多相互独立的进化途径, 测序技术水平的提高, 为雌雄异株进化过程的理论模型建立提供了较为可靠精细的支撑结果, 从而验证及突显了性别决定机制的多样性(Charlesworth & Charlesworth, 1978)。对不同雌雄异株物种的性别决定基因和候选基因进行初步的比较分析, 证实近缘的科、属物种有着不同的性别决定基因的起源, 以及相似的基因和途径被重复用于雌雄异株的独立进化, 尤其是细胞分裂素相关基因似乎在几个雌雄异株物种的性别决定中都十分重要。但目前大多数研究仅停留在鉴别出单个物种具体的某个或者某些性别决定基因, 缺乏后续功能验证和对应调控通路的解析, 需要进一步深入挖掘。新兴的HiFi和超长ONT三代测序技术, 以及基因编辑、多组学研究方法在近年来迅速成为鉴定性别决定基因和雌雄异株进化潜在遗传机制的热门技术, 未来几年必然会出现更多雌雄异株植物的性别决定相关研究结果, 研究重点可能会逐渐向性别决定基因的上下游基因以及整体调控通路方向延展。同时, 对于多次独立起源这一进化特征, 后续研究可以将聚焦点从单一物种逐步转向同科属物种, 或是具有相同、相近基因家族的性别决定基因以及同一调控通路上下游基因的物种, 从而扩大研究类群, 挖掘雌雄异株进化过程中的异同性, 解析基因组特征。随着更多物种研究结果的发表或许可以对现有模型进行补充和完善, 也可能会探究出新的植物性别研究模式物种。
植物性别决定机制和进化的研究不仅为染色体进化和花发育提供了生物学理论见解, 也可以为农业方面研发作物增产技术提供理论基础, 从而获得更高的经济收益。但目前对于雌雄异株植物性别鉴别方法的相关研究略显不足, 已有的雌雄个体物种鉴别手段大多是早期研发, 主要是利用外形、代谢物等生理生化指标进行辨别, 且部分鉴别方法易受个体和环境差异影响。近期的成果多为理论基础研究, 仅有极少数的物种, 获得了合适的分子标记应用于区分不同的雌雄个体。未来的研究方向应当将理论成果多与实际生产相结合, 积极开发和推广雌雄个体筛选的分子标记技术以及其他鉴别技术的应用。
参考文献
Cytological studies in the cycads: Sex chromosomes in Cycas
DOI:10.1093/oxfordjournals.aob.a083792 URL [本文引用: 1]
Epigenetic regulation of the sex determination gene MeGI in polyploid persimmon
DOI:10.1105/tpc.16.00532 URL [本文引用: 1]
A Y-encoded suppressor of feminization arose via lineage-specific duplication of a cytokinin response regulator in kiwifruit
DOI:10.1105/tpc.17.00787 URL [本文引用: 5]
Plant genetics. A Y-chromosome-encoded small RNA acts as a sex determinant in persimmons
DOI:10.1126/science.1257225 URL [本文引用: 2]
Two Y-chromosome-encoded genes determine sex in kiwifruit
DOI:10.1038/s41477-019-0489-6 URL [本文引用: 5]
The persimmon genome reveals clues to the evolution of a lineage-specific sex determination system in plants
Sex determination in flowering plants: Papaya as a model system
DOI:10.1016/j.plantsci.2013.10.018 URL [本文引用: 1]
The formation of sex chromosomes in Silene latifolia and S. dioica was accompanied by multiple chromosomal rearrangements
DOI:10.3389/fpls.2020.00205
PMID:32180787
[本文引用: 1]

The genus includes a plethora of dioecious and gynodioecious species. Two species, (white campion) and (red campion), are dioecious plants, having heteromorphic sex chromosomes with an XX/XY sex determination system. The X and Y chromosomes differ mainly in size, DNA content and posttranslational histone modifications. Although it is generally assumed that the sex chromosomes evolved from a single pair of autosomes, it is difficult to distinguish the ancestral pair of chromosomes in related gynodioecious and hermaphroditic plants. We designed an oligo painting probe enriched for X-linked scaffolds from currently available genomic data and used this probe on metaphase chromosomes of (2n = 24, XY), (2n = 24, XY), and two gynodioecious species, (2n = 24) and S. (2n = 24). The X chromosome-specific oligo probe produces a signal specifically on the X and Y chromosomes in and, mainly in the subtelomeric regions. Surprisingly, in and, the probe hybridized to three pairs of autosomes labeling their p-arms. This distribution suggests that sex chromosome evolution was accompanied by extensive chromosomal rearrangements in studied dioecious plants.Copyright © 2020 Bačovský, Čegan, Šimoníková, Hřibová and Hobza.
Structure and evolution of Apetala3, a sex-linked gene in Silene latifolia
DOI:10.1186/1471-2229-10-180 URL [本文引用: 1]
The evolution of sex chromosomes
Structurally distinct sex chromosomes (X and Y) are the most familiar mode of genetic sex determination and have evolved independently in many different taxa. The evolutionary paths by which their characteristic properties may have evolved are reviewed. These properties include the failure of X and Y to recombine through much or all of their length, the genetic inertness of much of the Y chromosome, dosage compensation of the activity of X chromosomal loci, and the accumulation of repeated DNA sequences on the Y chromosome.
A model for the evolution of dioecy and gynodioecy
DOI:10.1086/283342 URL [本文引用: 3]
Plant sex chromosome evolution
DOI:10.1093/jxb/ers322 URL [本文引用: 1]
Evolution of Sex Chromosomes in Plants. In:Encyclopedia of Life Sciences
Plant contributions to our understanding of sex chromosome evolution
DOI:10.1111/nph.13497
PMID:26053356
[本文引用: 4]

A minority of angiosperms have male and female flowers separated in distinct individuals (dioecy), and most dioecious plants do not have cytologically different (heteromorphic) sex chromosomes. Plants nevertheless have several advantages for the study of sex chromosome evolution, as genetic sex determination has evolved repeatedly and is often absent in close relatives. I review sex-determining regions in non-model plant species, which may help us to understand when and how (and, potentially, test hypotheses about why) recombination suppression evolves within young sex chromosomes. I emphasize high-throughput sequencing approaches that are increasingly being applied to plants to test for non-recombining regions. These data are particularly illuminating when combined with sequence data that allow phylogenetic analyses, and estimates of when these regions evolved. Together with comparative genetic mapping, this has revealed that sex-determining loci and sex-linked regions evolved independently in many plant lineages, sometimes in closely related dioecious species, and often within the past few million years. In reviewing recent progress, I suggest areas for future work, such as the use of phylogenies to allow the informed choice of outgroup species suitable for inferring the directions of changes, including testing whether Y chromosome-like regions are undergoing genetic degeneration, a predicted consequence of losing recombination.© 2015 The Authors. New Phytologist © 2015 New Phytologist Trust.
Plant sex chromosomes
DOI:10.1146/annurev-arplant-043015-111911
PMID:26653795
[本文引用: 1]

Although individuals in most flowering plant species, and in many haploid plants, have both sex functions, dioecious species-in which individuals have either male or female functions only-are scattered across many taxonomic groups, and many species have genetic sex determination. Among these, some have visibly heteromorphic sex chromosomes, and molecular genetic studies are starting to uncover sex-linked markers in others, showing that they too have fully sex-linked regions that are either too small or are located in chromosomes that are too small to be cytologically detectable from lack of pairing, lack of visible crossovers, or accumulation of heterochromatin. Detailed study is revealing that, like animal sex chromosomes, plant sex-linked regions show evidence for accumulation of repetitive sequences and genetic degeneration. Estimating when recombination stopped confirms the view that many plants have young sex-linked regions, making plants of great interest for studying the timescale of these changes.
Young sex chromosomes in plants and animals
DOI:10.1111/nph.16002
PMID:31222890
[本文引用: 1]

A major reason for studying plant sex chromosomes is that they may often be 'young' systems. There is considerable evidence for the independent evolution of separate sexes within plant families or genera, in some cases showing that the maximum possible time during which their sex-determining genes have existed must be much shorter than those of several animal taxa. Consequently, their sex-linked regions could either have evolved soon after genetic sex determination arose or considerably later. Plants, therefore, include species with both young and old systems. I review several questions about the evolution of sex-determining systems and sex chromosomes that require studies of young systems, including: the kinds of mutations involved in the transition to unisexual reproduction from hermaphroditism or monoecy (a form of functional hermaphroditism); the times when they arose; and the extent to which the properties of sex-linked regions of genomes reflect responses to new selective situations created by the presence of a sex-determining locus. I also evaluate which questions are best studied in plants, vs other suitable candidate organisms. Studies of young plant systems can help understand general evolutionary processes that are shared with the sex chromosomes of other organisms.© 2019 The Author. New Phytologist © 2019 New Phytologist Trust.
Steps in the evolution of heteromorphic sex chromosomes
DOI:10.1038/sj.hdy.6800697
PMID:15931241
[本文引用: 2]

We review some recently published results on sex chromosomes in a diversity of species. We focus on several fish and some plants whose sex chromosomes appear to be 'young', as only parts of the chromosome are nonrecombining, while the rest is pseudoautosomal. However, the age of these systems is not yet very clear. Even without knowing what proportions of their genes are genetically degenerate, these cases are of great interest, as they may offer opportunities to study in detail how sex chromosomes evolve. In particular, we review evidence that recombination suppression occurs progressively in evolutionarily independent cases, suggesting that selection drives loss of recombination over increasingly large regions. We discuss how selection during the period when a chromosome is adapting to its role as a Y chromosome might drive such a process.
The genomic architecture of sexual dimorphism in the dioecious plant Silene latifolia
Sex determination by two Y-linked genes in garden Asparagus
DOI:10.1105/tpc.19.00859
PMID:32220850
[本文引用: 3]

The origin and early evolution of sex chromosomes have been hypothesized to involve the linkage of factors with antagonistic effects on male and female function. Garden asparagus () is an ideal species to investigate this hypothesis, as the X and Y chromosomes are cytologically homomorphic and evolved from an ancestral autosome pair in association with a shift from hermaphroditism to dioecy. Mutagenesis screens paired with single-molecule fluorescence in situ hybridization directly implicate Y-specific genes that respectively suppress female (pistil) development and are necessary for male (anther) development. Comparison of contiguous X and Y chromosome assemblies shows that hemizygosity underlies the loss of recombination between the genes suppressing female organogenesis () and promoting male function ( []). We also experimentally demonstrate the function of These findings provide direct evidence that sex chromosomes can function through linkage of two sex determination genes.© 2020 American Society of Plant Biologists. All rights reserved.
The asparagus genome sheds light on the origin and evolution of a young Y chromosome
DOI:10.1038/s41467-017-01064-8
PMID:29093472
[本文引用: 6]

Sex chromosomes evolved from autosomes many times across the eukaryote phylogeny. Several models have been proposed to explain this transition, some involving male and female sterility mutations linked in a region of suppressed recombination between X and Y (or Z/W, U/V) chromosomes. Comparative and experimental analysis of a reference genome assembly for a double haploid YY male garden asparagus (Asparagus officinalis L.) individual implicates separate but linked genes as responsible for sex determination. Dioecy has evolved recently within Asparagus and sex chromosomes are cytogenetically identical with the Y, harboring a megabase segment that is missing from the X. We show that deletion of this entire region results in a male-to-female conversion, whereas loss of a single suppressor of female development drives male-to-hermaphrodite conversion. A single copy anther-specific gene with a male sterile Arabidopsis knockout phenotype is also in the Y-specific region, supporting a two-gene model for sex chromosome evolution.
Identification of a RAPD marker linked to sex determination in Pistacia vera using bulked segregant analysis
DOI:10.1007/BF00226975
PMID:24177762
[本文引用: 1]

The Random Amplified Polymorphic DNA (RAPD) technique was used to amplify DNA segments, with the objective of finding markers linked to sex determination in the dioecious species, Pistacia vera. Progenies from two female parents pollinated by a common male parent were studied. Two bulks of DNA were made in each cross, one from males and one from females, by pooling an equal weight of fresh leaves from each individual contributing to the bulk prior to DNA extraction. DNA was extracted from each bulked sample and from each of the contributing individuals. DNA was also extracted from 14 cultivars of P. vera and from 94 open-pollinated, fewweeks-old P. vera seedlings of unknown sex. Seven hundred different decamer oligonucleotide primers were used to perform DNA amplification, with 1 of these (OPO08) producing a 945 bp amplification band that was present only in the bulked female samples and absent in the bulked male samples of the two crosses. The relationship between band presence and female sex expression was conserved in every individual obtained from the two crosses and in the 14 cultivars unrelated to the crosses. We propose that this band is tightly linked to the gene(s) that control sex determination in pistachio. The OPO08945 RAPD marker could be used in a breeding program to screen the gender of pistachio plants long before they reach reproductive maturity, resulting in considerable savings of time and economic resources. In order to verify that assumption we screened 94 additional seedlings with the OPO08 primer and obtained results consistent with a 1∶1 male:female ratio.
A genetic study of the heterogametic nature of the staminate plant in spinach
The red bayberry genome and genetic basis of sex determination
DOI:10.1111/pbi.2019.17.issue-2 URL [本文引用: 1]
A new physical mapping approach refines the sex-determining gene positions on the Silene latifolia Y-chromosome
DOI:10.1038/srep18917 URL [本文引用: 1]
Sex identification of horticultural dioecious plants by phenolics analysis
酚类物质在鉴别园艺雌雄性植物中的应用研究
Fine mapping of the sex locus in Salix triandra confirms a consistent sex determination mechanism in genus Salix
DOI:10.1038/s41438-020-0289-1 URL [本文引用: 1]
The genomic architecture of the sex-determining region and sex-related metabolic variation in Ginkgo biloba
DOI:10.1111/tpj.v104.5 URL [本文引用: 1]
A primitive Y chromosome in papaya marks incipient sex chromosome evolution
DOI:10.1038/nature02228 URL [本文引用: 4]
DNA methylation and genetic degeneration of the Y chromosome in the dioecious plant Silene latifolia
DOI:10.1186/s12864-018-4936-y URL [本文引用: 1]
The genetic basis of sex determination in grapes
DOI:10.1038/s41467-020-16700-z
PMID:32518223
[本文引用: 2]

It remains a major challenge to identify the genes and mutations that lead to plant sexual differentiation. Here, we study the structure and evolution of the sex-determining region (SDR) in Vitis species. We report an improved, chromosome-scale Cabernet Sauvignon genome sequence and the phased assembly of nine wild and cultivated grape genomes. By resolving twenty Vitis SDR haplotypes, we compare male, female, and hermaphrodite haplotype structures and identify sex-linked regions. Coupled with gene expression data, we identify a candidate male-sterility mutation in the VviINP1 gene and potential female-sterility function associated with the transcription factor VviYABBY3. Our data suggest that dioecy has been lost during domestication through a rare recombination event between male and female haplotypes. This work significantly advances the understanding of the genetic basis of sex determination in Vitis and provides the information necessary to rapidly identify sex types in grape breeding programs.
Sex chromosomes in land plants
DOI:10.1146/annurev-arplant-042110-103914
PMID:21526970
[本文引用: 5]

Sex chromosomes in land plants can evolve as a consequence of close linkage between the two sex determination genes with complementary dominance required to establish stable dioecious populations, and they are found in at least 48 species across 20 families. The sex chromosomes in hepatics, mosses, and gymnosperms are morphologically heteromorphic. In angiosperms, heteromorphic sex chromosomes are found in at least 19 species from 4 families, while homomorphic sex chromosomes occur in 20 species from 13 families. The prevalence of the XY system found in 44 out of 48 species may reflect the predominance of the evolutionary pathway from gynodioecy towards dioecy. All dioecious species have the potential to evolve sex chromosomes, and reversions back from dioecy to various forms of monoecy, gynodioecy, or androdioecy have also occurred. Such reversals may occur especially during the early stages of sex chromosome evolution before the lethality of the YY (or WW) genotype is established.
Sex determination in papaya
Sex determination is an intriguing system in trioecious papaya. Over the past seven decades various hypotheses, based on the knowledge and information available at the time, have been proposed to explain the genetics of the papaya's sex determination. These include a single gene with three alleles, a group of closely linked genes, a genic balance of sex chromosome over autosomes, classical XY chromosomes, and regulatory elements of the flower development pathway. Recent advancements in genomic technology make it possible to characterize the genomic region involved in sex determination at the molecular level. High density linkage mapping validated the hypothesis that predicted recombination suppression at the sex determination locus. Physical mapping and sample sequencing of the non-recombination region led to the conclusion that sex determination is controlled by a pair of primitive sex chromosomes with a small male-specific region (MSY) of the Y chromosome. We now postulate that two sex determination genes control the sex determination pathway. One, a feminizing or stamen suppressor gene, causes stamen abortion before or at flower inception while the other, a masculinizing or carpel suppressor gene, causes carpel abortion at a later flower developmental stage. Detailed physical mapping is beginning to reveal structural details about the sex determination region and sequencing is expected to uncover candidate sex determining genes. Cloning of the sex determination genes and understanding the sex determination process could have profound application in papaya production.
A single gene underlies the dynamic evolution of poplar sex determination
DOI:10.1038/s41477-020-0672-9
PMID:32483326
[本文引用: 4]

Although hundreds of plant lineages have independently evolved dioecy (that is, separation of the sexes), the underlying genetic basis remains largely elusive. Here we show that diverse poplar species carry partial duplicates of the ARABIDOPSIS RESPONSE REGULATOR 17 (ARR17) orthologue in the male-specific region of the Y chromosome. These duplicates give rise to small RNAs apparently causing male-specific DNA methylation and silencing of the ARR17 gene. CRISPR-Cas9-induced mutations demonstrate that ARR17 functions as a sex switch, triggering female development when on and male development when off. Despite repeated turnover events, including a transition from the XY system to a ZW system, the sex-specific regulation of ARR17 is conserved across the poplar genus and probably beyond. Our data reveal how a single-gene-based mechanism of dioecy can enable highly dynamic sex-linked regions and contribute to maintaining recombination and integrity of sex chromosomes.
A small XY chromosomal region explains sex determination in wild dioecious V. vinifera and the reversal to hermaphroditism in domesticated grapevines
DOI:10.1186/s12870-014-0229-z URL [本文引用: 1]
An efficient RNA-seq-based segregation analysis identifies the sex chromosomes of Cannabis sativa
DOI:10.1101/gr.251207.119
PMID:32033943
[本文引用: 3]

-derived tetrahydrocannabinol (THC) production is increasing very fast worldwide. is a dioecious plant with XY Chromosomes, and only females (XX) are useful for THC production. Identifying the sex chromosome sequence would improve early sexing and better management of this crop; however, the genome projects have failed to do so. Moreover, as dioecy in the Cannabaceae family is ancestral, sex chromosomes are potentially old and thus very interesting to study, as little is known about old plant sex chromosomes. Here, we RNA-sequenced a family (two parents and 10 male and female offspring, 576 million reads) and performed a segregation analysis for all genes using the probabilistic method SEX-DETector. We identified >500 sex-linked genes. Mapping of these sex-linked genes to a genome assembly identified the largest chromosome pair being the sex chromosomes. We found that the X-specific region (not recombining between X and Y) is large compared to other plant systems. Further analysis of the sex-linked genes revealed that has a strongly degenerated Y Chromosome and may represent the oldest plant sex chromosome system documented so far. Our study revealed that old plant sex chromosomes can have large, highly divergent nonrecombining regions, yet still be roughly homomorphic.© 2020 Prentout et al.; Published by Cold Spring Harbor Laboratory Press.
Dioecy and its correlates in the flowering plants
DOI:10.1002/j.1537-2197.1995.tb11504.x URL [本文引用: 1]
Refined mapping of the Pierce’s disease resistance locus, PdR1, and sex on an extended genetic map of Vitis rupestris × V. arizonica
A framework genetic map based on genomic DNA-derived SSR, EST-derived SSR, EST-STS and EST-RFLP markers was developed using 181 genotypes generated from D8909-15 (female) x F8909-17 (male), the '9621' population. Both parents are half siblings with a common female parent, Vitis rupestris 'A. de Serres', and different male parents (forms of V. arizonica). A total of 542 markers were tested, and 237 of them were polymorphic for the female and male parents. The female map was developed with 159 mapped markers covering 865.0 cM with an average marker distance of 5.4 cM in 18 linkage groups. The male map was constructed with 158 mapped molecular markers covering 1055.0 cM with an average distance of 6.7 cM in 19 linkage groups. The consensus '9621' map covered 1154.0 cM with 210 mapped molecular markers in 19 linkage groups, with average distance of 5.5 cM. Ninety-four of the 210 markers on the consensus map were new. The 'Sex' expression locus segregated as single major gene was mapped to linkage group 2 on the consensus and the male map. PdR1, a major gene for resistance to Pierce's disease, caused by the bacterium Xylella fastidiosa, was mapped to the linkage group 14 between markers VMCNg3h8 and VVIN64, located 4.3 and 2.7 cM away from PdR1, respectively. Differences in segregation distortion of markers were also compared between parents, and three clusters of skewed markers were observed on linkage groups 6, 7 and 14.
Identification of a male-specific region (MSR) in Spinacia oleracea
DOI:10.1016/j.hpj.2021.01.003 URL [本文引用: 3]
A genetic linkage map of papaya based on randomly amplified polymorphic DNA markers
DOI:10.1007/BF00417946
PMID:24162346
[本文引用: 2]

A genetic linkage map of papaya (Carica papaya L.) was constructed using randomly amplified polymorphic DNA (RAPD) markers and a F2 population derived from a University of Hawaii UH breeding line 356 x 'Sunrise' cross. A total of 596 10-mer primers were screened, and 96 polymorphisms were detected. At LOD 4.0, 62 of these markers mapped to 11 linkage groups comprising 999.3 cM. About 80% of the markers conformed to expected Mendelian segregation ratios. We have mapped the locus that determines sex to a 14-cM region flanked by RAPD markers. The results demonstrate the usefulness of RAPD markers for developing a basic genetic linkage map in papaya.
Genetic mapping of sex determination in a wild strawberry, Fragaria virginiana, reveals earliest form of sex chromosome
DOI:10.1038/hdy.2008.100
PMID:18797475
[本文引用: 2]

The evolution of separate sexes (dioecy) from hermaphroditism is one of the major evolutionary transitions in plants, and this transition can be accompanied by the development of sex chromosomes. Studies in species with intermediate sexual systems are providing unprecedented insight into the initial stages of sex chromosome evolution. Here, we describe the genetic mechanism of sex determination in the octoploid, subdioecious wild strawberry, Fragaria virginiana Mill., based on a whole-genome simple sequence repeat (SSR)-based genetic map and on mapping sex determination as two qualitative traits, male and female function. The resultant total map length is 2373 cM and includes 212 markers on 42 linkage groups (mean marker spacing: 14 cM). We estimated that approximately 70 and 90% of the total F. virginiana genetic map resides within 10 and 20 cM of a marker on this map, respectively. Both sex expression traits mapped to the same linkage group, separated by approximately 6 cM, along with two SSR markers. Together, our phenotypic and genetic mapping results support a model of gender determination in subdioecious F. virginiana with at least two linked loci (or gene regions) with major effects. Reconstruction of parental genotypes at these loci reveals that both female and hermaphrodite heterogamety exist in this species. Evidence of recombination between the sex-determining loci, an important hallmark of incipient sex chromosomes, suggest that F. virginiana is an example of the youngest sex chromosome in plants and thus a novel model system for the study of sex chromosome evolution.
Genetics of the papaya
DOI:10.1093/oxfordjournals.jhered.a106358 URL [本文引用: 1]
Morphological characteristics and physiological-biochemical indexes of male and female Cercidiphyllum japonicum
连香树雌雄株叶片形态及生理生化指标比较
Repeated translocation of a gene cassette drives sex-chromosome turnover in strawberries
DOI:10.1371/journal.pbio.2006062 URL [本文引用: 1]
Correlates of monoicy and dioicy in hornworts, the apparent sister group to vascular plants
DOI:10.1186/1471-2148-13-239
PMID:24180692
[本文引用: 1]

Background: Whether male and female gametes are produced by single or separate individuals shapes plant mating and hence patterns of genetic diversity among and within populations. Haploid-dominant plants ("bryophytes": liverworts, mosses and hornworts) can have unisexual (dioicous) or bisexual (monoicous) gametophytes, and today, 68% of liverwort species, 57% of moss species, and 40% of hornwort species are dioicous. The transitions between the two sexual systems and possible correlations with other traits have been studied in liverworts and mosses, but not hornworts. Here we use a phylogeny for 98 of the 200 species of hornworts, the sister group to vascular plants, representing roughly equal proportions of all monoicous and all dioicous species, to test whether transitions in sexual systems are predominantly from monoicy to dioicy as might be expected based on studies of mosses. We further investigate possible correlations between sexual system and spore size, antheridium number, ploidy level, and diversification rate, with character selection partly based on findings in mosses and liverworts.;Results: Hornworts underwent numerous transitions between monoicy and dioicy. The transition rate from dioicy to monoicy was 2x higher than in the opposite direction, but monoicous groups have higher extinction rates; diversification rates do not correlate with sexual system. A correlation important in mosses, that between monoicy and polyploidy, apparently plays a small role: of 20 species with chromosome counts, only one is polyploid, the monoicous Anthoceros punctatus. A contingency test revealed that transitions to dioicy were more likely in species with small spores, supporting the hypothesis that small but numerous spores may be advantageous for dioicous species that depend on dense carpets of gametophytes for reproductive assurance. However, we found no evidence for increased antheridium-per-chamber numbers in dioicous species.;Conclusions: Sexual systems in hornworts are labile, and the higher number of extant monoicous species (60%) may be largely due to frequent transitions to monoicy.
Sexual systems in gymnosperms: A review
DOI:10.1016/j.baae.2018.05.009 URL [本文引用: 1]
Seasonal variation of endogenous hormones and nucleicacids in female and male plants of Ginko biloba
银杏雌雄株内源激素和核酸的变化
Sequencing papaya X and Yh chromosomes reveals molecular basis of incipient sex chromosome evolution
Sex determination, sex ratios, and genetic conflict
DOI:10.1146/ecolsys.1998.29.issue-1 URL [本文引用: 2]
The mechanism of sex determination in dioecious flowering plants
Gene organization of the liverwort Y chromosome reveals distinct sex chromosome evolution in a haploid system
A general model to explain repeated turnovers of sex determination in the Salicaceae
DOI:10.1093/molbev/msaa261 URL [本文引用: 4]
A willow sex chromosome reveals convergent evolution of complex palindromic repeats
DOI:10.1186/s13059-020-1952-4
PMID:32059685
[本文引用: 1]

Sex chromosomes have arisen independently in a wide variety of species, yet they share common characteristics, including the presence of suppressed recombination surrounding sex determination loci. Mammalian sex chromosomes contain multiple palindromic repeats across the non-recombining region that show sequence conservation through gene conversion and contain genes that are crucial for sexual reproduction. In plants, it is not clear if palindromic repeats play a role in maintaining sequence conservation in the absence of homologous recombination.Here we present the first evidence of large palindromic structures in a plant sex chromosome, based on a highly contiguous assembly of the W chromosome of the dioecious shrub Salix purpurea. The W chromosome has an expanded number of genes due to transpositions from autosomes. It also contains two consecutive palindromes that span a region of 200 kb, with conspicuous 20-kb stretches of highly conserved sequences among the four arms that show evidence of gene conversion. Four genes in the palindrome are homologous to genes in the sex determination regions of the closely related genus Populus, which is located on a different chromosome. These genes show distinct, floral-biased expression patterns compared to paralogous copies on autosomes.The presence of palindromes in sex chromosomes of mammals and plants highlights the intrinsic importance of these features in adaptive evolution in the absence of recombination. Convergent evolution is driving both the independent establishment of sex chromosomes as well as their fine-scale sequence structure.
Premature arrest of the male flower meristem precedes sexual dimorphism in the dioecious plant Silene latifolia