



Biodiv Sci ›› 2012, Vol. 20 ›› Issue (3): 250-263. DOI: 10.3724/SP.J.1003.201214027 cstr: 32101.14.SP.J.1003.201214027
Special Issue: 传粉生物学
• Reviews • Previous Articles Next Articles
Shan Sun1, Zhiqiang Zhang2, Bo Zhang3, Yongping Yang2,*( )
)
Received:2012-01-18
Accepted:2012-04-25
Online:2012-05-20
Published:2012-05-09
Contact:
Yongping Yang
Shan Sun, Zhiqiang Zhang, Bo Zhang, Yongping Yang. Perspectives on plant-pollinator interactions from the evolution of cooperation[J]. Biodiv Sci, 2012, 20(3): 250-263.
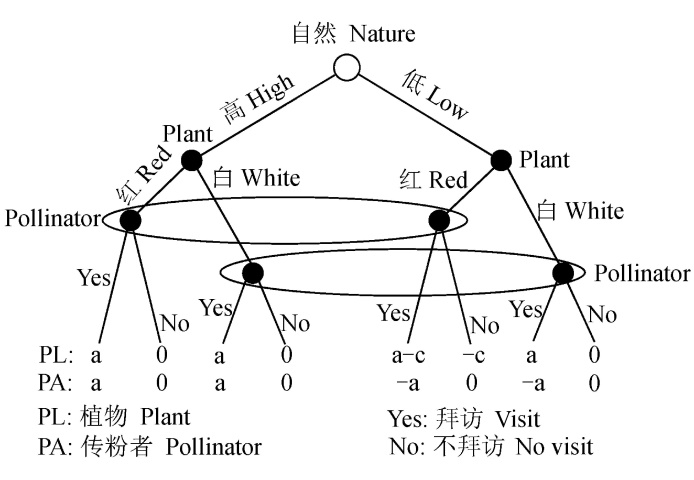
Fig. 1 A plant-pollinator signalling game example. There are two plant types: High quality or Low quality; They can choose to produce Red flowers or White flowers, which act as signals of plant types. Low quality plants must pay the cost, c, if it produces red flowers. The pollinator (receiver) witnesses the floral colors and chooses one of two behaviors, i.e., either visit or no visit. The ellipses in the figure show that the receiver cannot distinguish between two types of plants according to the floral colors. The red flower is the honest signal for High quality plant when a-c <0. a is the benefit to plant and pollinator when pollinator visits plant; 0 is no benefit; —, benefit is negative. Modified from Enquist et al.(2010).
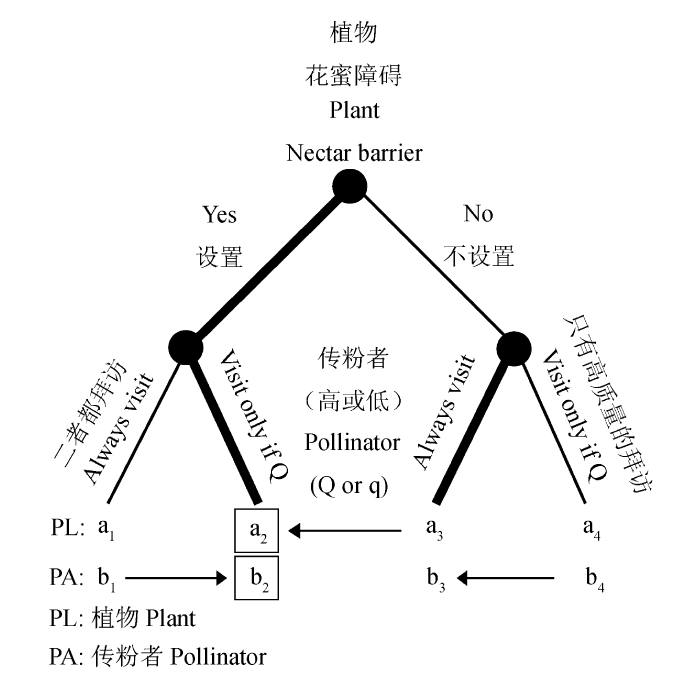
Fig. 2 A plant-pollinator screening game exmaple. There are two types of pollinators: good (Q) and bad (q) quality, which cannot be discriminated by plants. The plant can use nectar barrier to impose a cost for establishing the interaction, and the pollinator can “always visit” the plant (irrespective of being Q or q) or “visit only if Q”. The ai (a1< a3< a2< a4) and bi (b1< b2< b4< b3) show the payoffs to Plant (top) and Pollinator (bottom), respectively. Arrows show increases in payoff. The thick lines show the best strategies for each subgame. The boxes show the equilibrium solutions of the game. The best strategy for the plant is to use nectar barrier and for the pollinator to visit only when it is Q. Modified from Archetti et al.(2011a).
| [1] | Adler LS, Irwin RE (2005) Ecological costs and benefits of defenses in nectar. Ecology, 86, 2968-2978. |
| [2] |
Anderson B, Johnson SD, Carbutt C (2005) Exploitation of a specialized mutualism by a deceptive orchid. American Journal of Botany, 92, 1342-1349.
DOI URL PMID |
| [3] |
Archetti M, Scheuring I, Hoffman M, Frederickson ME, Pierce NE, Yu DW (2011a) Economic game theory for mutualism and cooperation. Ecology Letters, 14, 1300-1312.
DOI URL PMID |
| [4] | Archetti M, Úbeda F, Fudenberg D, Green J, Pierce NE, Yu DW (2011b) Let the right one in: a microeconomic approach to partner choice in mutualisms. The American Naturalist, 177, 75-85. |
| [5] | Armbruster WS, Antonsen L, Pélabon C (2005) Phenotypic selection on Dalechampia blossoms: honest signaling affects pollination success. Ecology, 86, 3323-3333. |
| [6] | Ashman TL, Stanton ML (1991) Seasonal variation in pollination dynamics of sexually dimorphic Sidalcea oregana ssp. spicata (Malvaceae). Ecology, 72, 993-1003. |
| [7] |
Axelrod R, Hamilton WD (1981) The evolution of cooperation. Science, 211, 1390-1396.
DOI URL PMID |
| [8] | Bascompte J, Jordano P (2007) Plant-animal mutualistic networks: the architecture of biodiversity. Annual Review of Ecology, Evolution, and Systematics, 38, 567-593. |
| [9] | Bell G (1986) The evolution of empty flowers. Journal of Theoretical Biology, 118, 253-258. |
| [10] |
Benitez-Vieyra S, Ordano M, Fornoni J, Boege K, Domínguez CA (2010) Selection on signal-reward correlation: limits and opportunities to the evolution of deceit in Turnera ulmifolia L. Journal of Evolutionary Biology, 23, 2760-2767.
URL PMID |
| [11] | Bernardello G, Anderson GJ, Stuessy TF, Crawford DJ (2001) A survey of floral traits, breeding systems, floral visitors, and pollination systems of the angiosperms of the Juan Fernandez Islands (Chile). Botanical Review, 67, 255-308. |
| [12] |
Biernaskie JM, Walker SC, Gegear RJ (2009) Bumblebees learn to forage like Bayesians. The American Naturalist, 174, 413-423.
URL PMID |
| [13] | Blarer A, Keasar T, Shmida A (2002) Possible mechanisms for the formation of flower size preferences by foraging bumblebees. Ethology, 108, 341-351. |
| [14] | Bolstad GH, Armbruster WS, Pélabon C, Pérez-Barrales R, Hansen TF (2010) Direct selection at the blossom level on floral reward by pollinators in a natural population of Dalechampia schottii: full-disclosure honesty? New Phytologist, 188, 370-384. |
| [15] | Brantjes NBM (1981) Floral mechanics in Phlomis (Lamiaceae). Annals of Botany, 47, 279-282. |
| [16] |
Bronstein JL (2001) The exploitation of mutualisms. Ecology Letters, 4, 277-287.
DOI URL |
| [17] |
Bull JJ, Rice WR (1991) Distinguishing mechanisms for the evolution of co-operation. Journal of Theoretical Biology, 149, 63-74.
URL PMID |
| [18] | Cartar RV (2004) Resource-tracking by bumble bees: responses to plant-level differences in quality. Ecology, 85, 2764-2771. |
| [19] |
Castellanos MC, Wilson P, Thomson JD (2004) ‘Anti-bee’ and ‘pro-bird’ changes during the evolution of hummingbird pollination in Penstemon flowers. Journal of Evolutionary Biology, 17, 876-885.
URL PMID |
| [20] |
Chittka L, Schürkens S (2001) Successful invasion of a floral market. Nature, 411, 653.
DOI URL PMID |
| [21] | Claßen-Bockhoff R, Speck T, Tweraser E, Wester P, Thimm S, Reith M (2004) The staminal lever mechanism in Salvia L. (Lamiaceae): a key innovation for adaptive radiation? Organisms, Diversity and Evolution, 4, 189-205. |
| [22] |
Córdoba SA, Cocucci AA (2011) Flower power: its association with bee power and floral functional morphology in papilionate legumes. Annals of Botany, 108, 919-931.
DOI URL PMID |
| [23] | Culley TM, Weller SG, Sakai AK (2002) The evolution of wind pollination in angiosperms. Trends in Ecology and Evolution, 17, 361-369. |
| [24] | de Jong TL, Klinkhamer PGL(2005) Evolutionary Ecology of Plant Reproductive Strategies. Cambridge University Press, Cambridge. |
| [25] | Dedej S, Delaplane KS (2005) Net energetic advantage drives honey bees (Apis mellifera L.) to nectar larceny in Vacciniumashei Reade. Behavioral Ecology and Sociobiology, 57, 398-403. |
| [26] |
Douglas AE (2008) Conflict, cheats and the persistence of symbioses. New Phytologist, 177, 849-858.
DOI URL |
| [27] |
Eckert CG, Kalisz S, Geber MA, Sargent R, Elle E, Cheptou PO, Goodwillie C, Johnston MO, Kelly KJ, Moeller DA, Porcher E, Ree RH, Vallejo-Marin M, Winn AA (2010) Plant mating systems in a changing world. Trends in Ecology and Evolution, 25, 35-43.
URL PMID |
| [28] |
Edwards DP (2009) The roles of tolerance in the evolution, maintenance and breakdown of mutualism. Naturwissenschaften, 96, 1137-1145.
DOI URL PMID |
| [29] |
Edwards J, Whitaker D, Klionsky S, Laskowski MJ (2005) A record-breaking pollen catapult. Nature, 435, 164.
DOI URL PMID |
| [30] | Enquist M, Hurd PL, Ghirlanda S (2010) Signalling. In: Evolutionary Behavioral Ecology (eds Westneat DF, Fox CW), pp. 266-284. Oxford University Press, New York. |
| [31] |
Fenster CB, Cheely G, Dudash MR, Reynolds RJ (2006) Nectar reward and advertisement in hummingbird-pollinated Silene virginica (Caryophyllaceae). American Journal of Botany, 93, 1800-1807.
DOI URL PMID |
| [32] |
Ferrière R, Gauduchon M, Bronstein JL (2007) Evolution and persistence of obligate mutualists and exploiters: competition for partners and evolutionary immunization. Ecology Letters, 10, 115-126.
DOI URL PMID |
| [33] |
Fishman MA, Hadany L (2010) Plant-pollinator population dynamics. Theoretical Population Biology, 78, 270-277.
DOI URL PMID |
| [34] |
Forrest J, Thomson JD (2009) Pollinator experience, neophobia and the evolution of flowering time. Proceedings of the Royal Society B: Biological Sciences, 276, 935-943.
URL PMID |
| [35] |
Foster KR, Kokko H (2006) Cheating can stabilize cooperation in mutualisms. Proceedings of the Royal Society B: Biological Sciences, 273, 2233-2239.
DOI URL PMID |
| [36] | Franks SJ, Sim S, Weis AE (2007) Rapid evolution of flowering time by an annual plant in response to a climate fluctuation. Proceedings of the National Academy of Sciences, USA, 104, 1278-1282. |
| [37] |
Galen C, Kaczorowski R, Todd SL, Geib J, Raguso RA (2011) Dosage-dependent impacts of a floral volatile compound on pollinators, larcenists, and the potential for floral evolution in the alpine skypilot Polemonium viscosum. The American Naturalist, 177, 258-272.
DOI URL PMID |
| [38] |
Gao JY, Ren PY, Yang ZH, Li QJ (2006) The pollination ecology of Paraboea rufescens (Gesneriaceae): a buzz- pollinated tropical herb with mirror image flowers. Annals of Botany, 97, 371-376.
DOI URL PMID |
| [39] |
Gegear RJ, Manson JS, Thomson JD (2007) Ecological context influences pollinator deterrence by alkaloids in floral nectar. Ecology Letters, 10, 375-382.
DOI URL PMID |
| [40] |
Gilbert FS, Haines N, Dickson K (1991) Empty flowers. Functional Ecology, 5, 29-39.
DOI URL |
| [41] |
Golubov J, Eguiarte LE, Mandujano MC, López-Portillo J, Montaña C (1999) Why be a honeyless honey mesquite? Reproduction and mating system of nectarful and nectarless individuals. American Journal of Botany, 86, 955-963.
URL PMID |
| [42] |
Gómez JM, Bosch J, Perfectti F, Fernández JD, Abdelaziz M, Camacho JPM (2008) Association between floral traits and rewards in Erysimum mediohispanicum (Brassicaceae). Annals of Botany, 101, 1413-1420.
DOI URL PMID |
| [43] |
Gordo O, Sanz JJ (2005) Phenology and climate change: a long-term study in a Mediterranean locality. Oecologia, 146, 484-495.
DOI URL PMID |
| [44] |
Goto R, Okamoto T, Kiers ET, Kawakita A, Kato M (2010) Selective flower abortion maintains moth cooperation in a newly discovered pollination mutualism. Ecology Letters, 13, 321-329.
DOI URL PMID |
| [45] |
Gumbert A (2000) Color choices by bumble bees (Bombus terrestris): innate preferences and generalization after learning. Behavioral Ecology and Sociobiology, 48, 36-43.
DOI URL |
| [46] |
Hansen DM, Beer K, Müeller CB (2006) Mauritian coloured nectar no longer a mystery: a visual signal for lizard pollinators. Biology Letters, 2, 165-168.
DOI URL PMID |
| [47] | Harder LD, Barclay RMR (1994) The functional significance of poricidal anthers and buzz pollination: controlled pollen removal from Dodecatheon. Functional Ecology, 8, 509-517. |
| [48] |
Hargreaves AL, Harder LD, Johnson SD (2009) Consumptive emasculation: the ecological and evolutionary consequences of pollen theft. Biological Reviews, 84, 259-276.
DOI URL PMID |
| [49] | Harrington JE Jr (2009) Game, Strategies, and Decision Making. Worth Publishers, New York. |
| [50] |
Hegland SJ, Nielsen A, Lázaro A, Bjerknes AL, Totland Ø (2009) How does climate warming affect plant-pollinator interactions? Ecology Letters, 12, 184-195.
DOI URL PMID |
| [51] |
Holland JN, DeAngelis DL (2001) Population dynamics and the ecological stability of obligate pollination mutualisms. Oecologia, 126, 575-586.
DOI URL PMID |
| [52] | Howell AD, Alarcón R (2007) Osmia bees (Hymenoptera: Megachilidae) can detect nectar-rewarding flowers using olfactory cues. Animal Behaviour, 74, 199-205. |
| [53] |
Internicola AI, Harder LD (2012) Bumble-bee learning selects for both early and long flowering in food-deceptive plants. Proceedings of the Royal Society B: Biological Sciences, 279, 1538-1543.
DOI URL PMID |
| [54] | Internicola AI, Page PA, Bernasconi G, Gigord LDB (2007) Competition for pollinator visitation between deceptive and rewarding artificial inflorescences: an experimental test of the effects of floral colour similarity and spatial mingling. Functional Ecology, 21, 864-872. |
| [55] |
Irwin RE (2009) Realized tolerance to nectar robbing: compensation to floral enemies in Ipomopsis aggregata. Annals of Botany, 103, 1425-1433.
DOI URL PMID |
| [56] |
Irwin RE, Adler LS (2008) Nectar secondary compounds affect self-pollen transfer: implications for female and male reproduction. Ecology, 89, 2207-2217
DOI URL PMID |
| [57] | Irwin RE, Bronstein JL, Manson JS, Richardson L (2010) Nectar robbing: ecological and evolutionary perspectives. Annual Review of Ecology, Evolution, and Systematics, 41, 271-292. |
| [58] |
Irwin RE, Galen C, Rabenold JJ, Kaczorowski R, McCutcheon ML (2008) Mechanisms of tolerance to floral larceny in two wildflower species. Ecology, 89, 3093-3104.
DOI URL PMID |
| [59] |
Johnson SD, Hargreaves AL, Brown M (2006) Dark, bitter-tasting nectar functions as a filter of flower visitors in a bird-pollinated plant. Ecology, 87, 2709-2716.
DOI URL PMID |
| [60] |
Jokela J, Schmid-Hempel P, Rigby MC (2000) Dr. Pangloss restrained by the Red Queen―steps towards a unified defence theory. Oikos, 89, 267-274.
DOI URL |
| [61] | Kessler D, Baldwin IT (2007) Making sense of nectar scents: the effects of nectar secondary metabolites on floral visitors of Nicotiana attenuata. The Plant Journal, 46, 840-854. |
| [62] |
Kessler D, Diezel C, Baldwin IT (2010) Changing pollinators as a means of escaping herbivores. Current Biology, 20, 237-242.
DOI URL PMID |
| [63] |
Kessler D, Gase K, Baldwin IT (2008) Field experiments with transformed plants reveal the sense of floral scents. Science, 321, 1200-1202.
DOI URL PMID |
| [64] |
Kiers ET, Palmer TM, Ives AR, Bruno JF, Bronstein JL (2010) Mutualisms in a changing world: an evolutionary perspective. Ecology Letters, 13, 1459-1474.
DOI URL PMID |
| [65] |
Klinkhamer PGL, de Jong TL, Linnebank LA (2001) Small-scale spatial patterns determine ecological relationships: an experimental example using nectar production rates. Ecology Letters, 4, 559-567.
DOI URL |
| [66] |
Kudo G, Nishikawa Y, Kasagi T, Kosuge S (2004) Does seed production of spring ephemerals decrease when spring comes early? Ecological Research, 19, 255-259.
DOI URL |
| [67] |
Leigh EG Jr (2010) The evolution of mutualism. Journal of Evolutionary Biology, 23, 2507-2528.
DOI URL PMID |
| [68] |
Leiss KA, Vrieling K, Klinkhamer PGL (2004) Heritability of nectar production in Echium vulgare. Heredity, 92, 446-451.
DOI URL PMID |
| [69] |
Lopezaraiza-Mikel ME, Hayes RB, Whalley MR, Memmott J(2007) The impact of an alien plant on a native plant- pollinator network: an experimental approach. Ecology Letters, 10, 539-550.
DOI URL PMID |
| [70] |
Luo Z, Zhang D, Renner SS (2008) Why two kinds of stamens in buzz-pollinated flowers? Experimental support for Darwin’s division-of-labour hypothesis. Functional Ecology, 22, 794-800.
DOI URL |
| [71] | Makino TT, Sakai S (2007) Experience changes pollinator responses to floral display size: from size-based to reward-based foraging. Functional Ecology, 21, 854-863. |
| [72] |
McDade LA, Kinsman S (1980) The impact of floral parasitism in two neotropical hummingbird pollinated plant species. Evolution, 34, 944-958.
DOI URL PMID |
| [73] |
Memmott J, Craze PG, Waser NM, Price MV (2007) Global warming and the disruption of plant-pollinator interactions. Ecology Letters, 10, 710-717.
DOI URL PMID |
| [74] | Møller AP, (1995) Bumblebee preference for symmetrical flowers. Proceedings of the National Academy of Sciences, USA, 92, 2288-2292. |
| [75] | Morris WF, Bronstein JL, Wilson WG (2003) Three-way coexistence in obligate mutualist-exploiter communities: the potential role of competition. The American Naturalist, 166, 860-875. |
| [76] | Morris WF, Vázquez DP, Chacoff NP (2010) Benefit and cost curves for typical pollination mutualisms. Ecology, 95, 1276-1285. |
| [77] |
Muchhala N, Brown Z, Armbruster WS, Potts MD (2011) Competition drives specialization in pollination systems through costs to male fitness. The American Naturalist, 176, 732-743.
DOI URL PMID |
| [78] |
Muñoz AA, Cavieres LA (2008) The presence of a showy invasive plant disrupts pollinator service and reproductive output in native alpine species only at high densities. Journal of Ecology, 96, 459-467.
DOI URL |
| [79] |
Nowak MA (2006) Five rules for the evolution of cooperation. Science, 314, 1560-1563.
DOI URL PMID |
| [80] |
Ohashi K (2002) Consequences of floral complexity for bumblebee-mediated geitonogamous self-pollination in Salvia nipponica Miq. (Labiatae). Evolution, 56, 2414-2423.
DOI URL PMID |
| [81] |
Oliver TH, Leather SR, Cook JM (2009) Tolerance traits and the stability of mutualism. Oikos, 118, 346-352.
DOI URL |
| [82] |
Pellmyr O, Huth CJ (1994) Evolutionary stability of mutualism between yuccas and yucca moths. Nature, 372, 257-260.
DOI URL |
| [83] |
Pellmyr O, Leebens-Mack J, Huth CJ (1996) Non-mutualistic yucca moths and their evolutionary consequences. Nature, 380, 155-156.
DOI URL PMID |
| [84] |
Pyke GH (1982) Local geographic distributions of bumblebees near Crested Butte, Colorado: competition and community structure. Ecology, 63, 555-573.
DOI URL |
| [85] | Riffell JA, Alarcon R, Abrell L, Davidowitz G, Bronstein JL, Hildebrand JG (2008) Behavioral consequences of innate preferences and olfactory learning in hawkmoth-flower interactions. Proceedings of the National Academy of Sciences, USA, 105, 3404-3409. |
| [86] |
Rodríguez-Gironés MA, Santamaría L (2007) Resource competition, character displacement, and the evolution of deep corolla tubes. The American Naturalist, 170, 455-464.
DOI URL PMID |
| [87] |
Sachs JL, Simms EL (2006) Pathways to mutualism breakdown. Trends in Ecology and Evolution, 21, 585-592.
DOI URL PMID |
| [88] |
Salzmann CC, Nardella AM, Cozzolino S, Schiestl FP (2007) Variability in floral scent in rewarding and deceptive orchids: the signature of pollinator-imposed selection? Annals of Botany, 100, 757-765.
DOI URL PMID |
| [89] |
Schaefer HM, Ruxton GD (2009) Deception in plants: mimicry or perceptual exploitation? Trends in Ecology and Evolution, 24, 676-685.
DOI URL PMID |
| [90] | Schaefer HM, Schmidt V, Levey DJ (2004) How plant-animal interactions signal new insights in communication. Trends in Ecology and Evolution, 19, 577-584. |
| [91] |
Smithson A, Gigord LDB (2003) The evolution of empty flowers revisited. The American Naturalist, 161, 537-552.
DOI URL PMID |
| [92] |
Traveset A, Richardson DM (2006) Biological invasions as disruptors of plant reproductive mutualisms. Trends in Ecology and Evolution, 21, 208-216.
DOI URL PMID |
| [93] |
Wang YS, Wu H, Sun S (2012) Persistence of pollination mutualisms in plant-pollinator-robber systems. Theoretical Population Biology, 81, 243-250.
DOI URL PMID |
| [94] | Weiss MR (1991) Floral colour changes as cues for pollinators. Nature, 354, 227-229. |
| [95] |
West SA, Griffin AS, Gardner A (2007) Evolutionary explanations for cooperation. Current Biology, 17, R661-R672.
DOI URL PMID |
| [96] | Weyl EG, Frederickson ME, Yu DW, Pierce NE (2010) Economic contract theory tests models of mutualism. Proceedings of the National Academy of Sciences, USA, 107, 15712-15716. |
| [97] | Wilson WG, Morris WF, Bronstein JL (2003) Coexistence of mutualists and exploiters on spatial landscapes. Ecological Monographs, 73, 397-413. |
| [98] | Wright GA, Schiestl FP (2009) The evolution of floral scent: the influence of olfactory learning by insect pollinators on the honest signalling of floral rewards. Functional Ecology, 23, 841-851. |
| [99] | Yu DW (2001) Parasites of mutualisms. Biological Journal of the Linnean Society, 72, 529-546. |
| [100] |
Zhang B, Claßen-Bockhoff R, Zhang ZQ, Sun S, Luo YJ, Li QJ (2011a) Functional implications of the staminal lever mechanism in Salvia cyclostegia (Lamiaceae). Annals of Botany, 107, 621-628.
DOI URL PMID |
| [101] |
Zhang C, Irwin RE, Wang Y, He YP, Yang YP, Duan YW (2011b) Selective seed abortion induced by nectar robbing in the selfing plant C omastoma pulmonarium. New Phytologist, 192, 249-255.
DOI URL |
| [102] |
Zhang FP, Cai XH, Wang H, Ren ZX, Larson-Rabin Z, Li DZ (2012) Dark purple nectar as a foraging signal in a bird-pollinated Himalayan plant. New Phytologist, 193, 188-195.
DOI URL |
| [1] | Liu Li, Zang Mingyue, Ma Yue, Wan Yaqiong, Hu Feilong, Lu Xiaoqiang, Liu Yan. Measures, progress and prospects of central-local cooperation in the implementation of the National Biodiversity Strategy and Action Plan [J]. Biodiv Sci, 2025, 33(3): 24532-. |
| [2] | Rui Qu, Zhenjun Zuo, Youxin Wang, Liangjian Zhang, Zhigang Wu, Xiujuan Qiao, Zhong Wang. The biogeochemical niche based on elementome and its applications in different ecosystems [J]. Biodiv Sci, 2024, 32(4): 23378-. |
| [3] | Xiaobo Lü, Donghai Li, Xiaobo Yang, Mengwen Zhang. The species coexisted in mangrove communities through niche differentiation of flooding time and salinity [J]. Biodiv Sci, 2024, 32(3): 23302-. |
| [4] | Xintong Gong, Fei Chen, Huanhuan Gao, Xinqiang Xi. Larva and adult competition between two Drosophila species and the effects on species coexistence [J]. Biodiv Sci, 2023, 31(8): 22603-. |
| [5] | Xiang Liu, Mu Liu, Yao Xiao. The effect of foliar fungal pathogens on plant species coexistence: Progress and challenges [J]. Biodiv Sci, 2023, 31(2): 22525-. |
| [6] | Tian Luo, Fangyuan Yu, Juyu Lian, Junjie Wang, Jian Shen, Zhifeng Wu, Wanhui Ye. Impact of canopy vertical height on leaf functional traits in a lower subtropical evergreen broad-leaved forest of Dinghushan [J]. Biodiv Sci, 2022, 30(5): 21414-. |
| [7] | Nan Ye, Beibei Hou, Chao Wang, Ruiwu Wang, Jianxiao Song. Spatial self-organization in microbial interactions [J]. Biodiv Sci, 2022, 30(5): 21458-. |
| [8] | Shaopeng Wang, Mingyu Luo, Yanhao Feng, Chengjin Chu, Dayong Zhang. Theoretical advances in biodiversity research [J]. Biodiv Sci, 2022, 30(10): 22410-. |
| [9] | Zhilin Li, Li’an Duo, Sheng Li, Tianming Wang. Competition and coexistence among terrestrial mammalian carnivores [J]. Biodiv Sci, 2021, 29(1): 81-97. |
| [10] | Mingzhen Lu. Plant-microbe mutualism: Evolutionary mechanisms and ecological functions [J]. Biodiv Sci, 2020, 28(11): 1311-1323. |
| [11] | Chuliang Song. Structural stability: Concepts, methods, and applications [J]. Biodiv Sci, 2020, 28(11): 1345-1361. |
| [12] | Miao Wu, Yun Hao, Xiaoyun Zhang, Lixian Wang, Jingjing He, Guangzheng Duan. Biodiversity conservation of Kazakhstan and suggestions on cooperation between China and Kazakhstan [J]. Biodiv Sci, 2020, 28(10): 1276-1285. |
| [13] | Chen Lijun,Shu Zufei,Xiao Zhishu. Application of camera-trapping data to study daily activity patterns of Galliformes in Guangdong Chebaling National Nature Reserve [J]. Biodiv Sci, 2019, 27(3): 266-272. |
| [14] | Ruyun Zhang, Yanpeng Li, Yunlong Ni, Xujun Gui, Juyu Lian, Wanhui Ye. Intraspecific variation of leaf functional traits along the vertical layer in a subtropical evergreen broad-leaved forest of Dinghushan [J]. Biodiv Sci, 2019, 27(12): 1279-1290. |
| [15] | Wenjing Liu, Jing Xu, Senlu Yin, Yu Tian, Junsheng Li. Mechanisms of benefit-sharing of medicinal plants found in China and neighboring countries [J]. Biodiv Sci, 2017, 25(8): 907-913. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn ![]()