



生物多样性 ›› 2021, Vol. 29 ›› Issue (1): 43-52. DOI: 10.17520/biods.2019415 cstr: 32101.14.biods.2019415
所属专题: 土壤生物与土壤健康
收稿日期:2019-12-31
接受日期:2020-03-20
出版日期:2021-01-20
发布日期:2020-06-12
通讯作者:
张元明
基金资助:
Xin Liu1,2( ), Xiaoying Rong1(
), Xiaoying Rong1( ), Yuanming Zhang1,*(
), Yuanming Zhang1,*( )(
)( )
)
Received:2019-12-31
Accepted:2020-03-20
Online:2021-01-20
Published:2020-06-12
Contact:
Yuanming Zhang
摘要:
生物结皮作为荒漠地表的重要覆被类型, 在荒漠生态系统的氮素循环中扮演重要角色。融雪期为古尔班通古特沙漠生物结皮的复苏和生长提供了充足的水分, 也成为该沙漠氮素固定和转化的重要时期, 但该时期生物结皮如何影响驱动氨氧化转化的微生物群落动态尚未明确。因此, 我们利用荧光定量PCR (fluorescent quantitative PCR, qPCR)方法分析融雪期生物结皮与去除结皮不同土层(0-2, 2-5, 5-10和10-20 cm)氨氧化菌群丰度特征, 结合潜在硝化速率和土壤理化参数, 探究融雪期生物结皮对荒漠土壤氮素转化作用。结果表明: 氨氧化古菌(ammonia-oxidizing archaea, AOA)是古尔班通古特沙漠土壤优势氨氧化菌, 生物结皮对0-2 cm层土壤中AOA、氨氧化细菌(ammonia-oxidizing bacteria, AOB) amoA基因丰度具有显著抑制作用(P < 0.01), 对10-20 cm层土壤中AOA amoA基因丰度具有显著促进作用(P < 0.01)。冗余分析(redundancy analysis, RDA)表明, AOA、AOB amoA基因丰度主要受土壤含水量和铵态氮含量的影响, 占总条件效应的54.90%。氨氧化速率分析发现, 去除生物结皮显著降低古尔班通古特沙漠土壤硝化作用潜力(P < 0.001), 证实生物结皮对荒漠土壤氮素转化具有重要的调控作用。综上所述, 古尔班通古特沙漠氨氧化微生物的分布规律受环境因子调控, 特别是生物结皮可以通过调节土壤含水量和铵态氮含量影响AOA和AOB的空间生态位分化, 促进沙漠土壤的硝化作用。
刘鑫, 荣晓莹, 张元明 (2021) 古尔班通古特沙漠生物土壤结皮对氨氧化微生物生态位的影响. 生物多样性, 29, 43-52. DOI: 10.17520/biods.2019415.
Xin Liu, Xiaoying Rong, Yuanming Zhang (2021) Biocrusts impact niche separation of ammonia oxidizing microorganisms in the Gurbantunggut Desert, northwestern China. Biodiversity Science, 29, 43-52. DOI: 10.17520/biods.2019415.
| 温度 Temperature (℃) | 含水量 Soil moisture (%) | pH | 电导率 Conductivity (μs/cm) | 全碳 Total Carbon (mg/g) | 全氮 Total Nitrogen (mg/g) | |
|---|---|---|---|---|---|---|
| 结皮覆盖 Biocrusts | ||||||
| 0-2 cm | 0.5 ± 0.0 A** | 10.63 ± 0.45 A | 8.25 ± 0.05 C | 4.55 ± 0.26 A | 6.72 ± 0.12 A* | 0.38 ± 0.02 A** |
| 2-5 cm | 0.2 ± 0.0 B* | 6.17 ± 0.23 B** | 8.37 ± 0.03 B | 3.38 ± 0.17 B | 5.76 ± 0.17 A | 0.23 ± 0.02 B |
| 5-10 cm | 0.2 ± 0.0a B** | 3.90 ± 0.12 C | 8.55 ± 0.02 A | 2.92 ± 0.04 B** | 6.74 ± 0.29 A | 0.25 ± 0.01 B |
| 10-20 cm | 0.1 ± 0.0 B** | 2.00 ± 0.21 D | 8.57 ± 0.01 A | 3.33 ± 0.16 B** | 6.47 ± 0.33 A | 0.23 ± 0.02 B |
| 去除结皮 Biocrusts-removal | ||||||
| 0-2 cm | 0.1 ± 0.0 A | 10.75 ± 1.54 A | 8.43 ± 0.06 A* | 3.96 ± 0.53 A | 5.86 ± 0.21 A | 0.23 ± 0.01 AB |
| 2-5 cm | 0.1 ± 0.0 A | 4.90 ± 0.21 B | 8.44 ± 0.02 A | 3.65 ± 0.24 A | 5.83 ± 0.39 A | 0.27 ± 0.00 A* |
| 5-10 cm | -0.1 ± 0.0a B | 3.65 ± 0.22 B | 8.60 ± 0.05 A | 2.37 ± 0.13 B | 5.82 ± 0.44 A | 0.27 ± 0.02 AB |
| 10-20 cm | -0.2 ± 0.0 C | 2.48 ± 0.10 B | 8.61 ± 0.07 A | 2.65 ± 0.06 B | 6.04 ± 0.23 A | 0.22 ± 0.01 B |
表1 土壤理化性质
Table 1 Soil physical and chemical properties
| 温度 Temperature (℃) | 含水量 Soil moisture (%) | pH | 电导率 Conductivity (μs/cm) | 全碳 Total Carbon (mg/g) | 全氮 Total Nitrogen (mg/g) | |
|---|---|---|---|---|---|---|
| 结皮覆盖 Biocrusts | ||||||
| 0-2 cm | 0.5 ± 0.0 A** | 10.63 ± 0.45 A | 8.25 ± 0.05 C | 4.55 ± 0.26 A | 6.72 ± 0.12 A* | 0.38 ± 0.02 A** |
| 2-5 cm | 0.2 ± 0.0 B* | 6.17 ± 0.23 B** | 8.37 ± 0.03 B | 3.38 ± 0.17 B | 5.76 ± 0.17 A | 0.23 ± 0.02 B |
| 5-10 cm | 0.2 ± 0.0a B** | 3.90 ± 0.12 C | 8.55 ± 0.02 A | 2.92 ± 0.04 B** | 6.74 ± 0.29 A | 0.25 ± 0.01 B |
| 10-20 cm | 0.1 ± 0.0 B** | 2.00 ± 0.21 D | 8.57 ± 0.01 A | 3.33 ± 0.16 B** | 6.47 ± 0.33 A | 0.23 ± 0.02 B |
| 去除结皮 Biocrusts-removal | ||||||
| 0-2 cm | 0.1 ± 0.0 A | 10.75 ± 1.54 A | 8.43 ± 0.06 A* | 3.96 ± 0.53 A | 5.86 ± 0.21 A | 0.23 ± 0.01 AB |
| 2-5 cm | 0.1 ± 0.0 A | 4.90 ± 0.21 B | 8.44 ± 0.02 A | 3.65 ± 0.24 A | 5.83 ± 0.39 A | 0.27 ± 0.00 A* |
| 5-10 cm | -0.1 ± 0.0a B | 3.65 ± 0.22 B | 8.60 ± 0.05 A | 2.37 ± 0.13 B | 5.82 ± 0.44 A | 0.27 ± 0.02 AB |
| 10-20 cm | -0.2 ± 0.0 C | 2.48 ± 0.10 B | 8.61 ± 0.07 A | 2.65 ± 0.06 B | 6.04 ± 0.23 A | 0.22 ± 0.01 B |
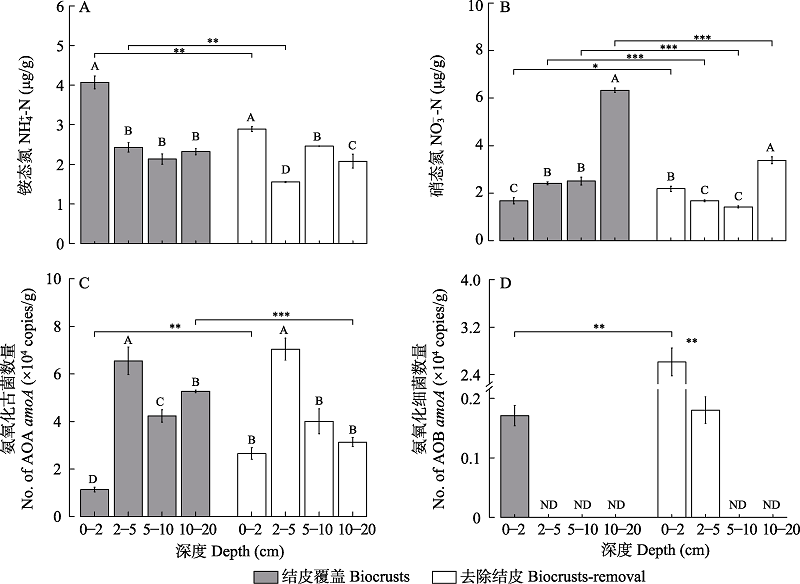
图1 古尔班通古特沙漠土壤有效态氮含量与氨氧化菌群丰度。ND表示amoA基因丰度低于检测标准。大写字母表示相同结皮处理不同土层显著差异性,α = 0.05。*为相同土层不同结皮处理之间差异性; * P < 0.05, ** P < 0.01, *** P < 0.001。
Fig. 1 Available nitrogen content and amoA gene abundance in the Gurbantunggut Desert during snow melt period. (A): NH4+-N (µg/g); (B): NO3--N (µg/g); (C): AOA amoA gene abundance (copies/g) and (D): AOB amoA gene abundance (copies/g). ND indicates that amoA gene abundance is lower than the detection empirical value. Capital letters indicate significant differences among soil depth in biocrusts or biocrusts-removal treatments, α = 0.05. * indicates the difference between biocrusts and biocrusts-removal treatments for the same soil depth; * P < 0.05, ** P < 0.01, *** P < 0.001
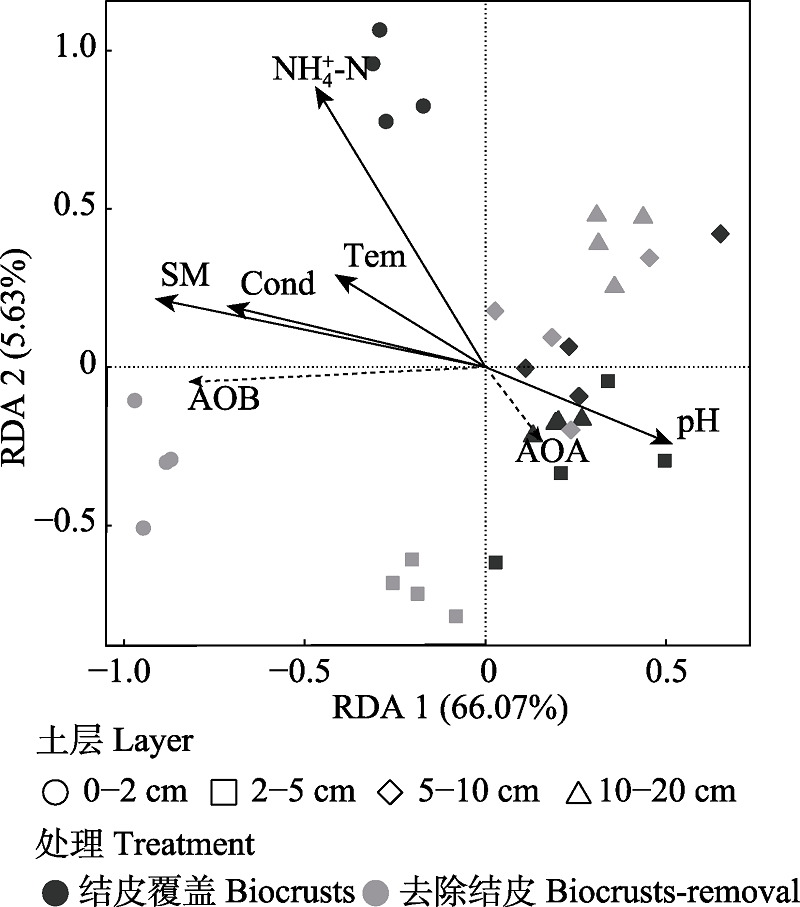
图2 AOA和AOB amoA基因丰度与环境参数的冗余分析。Tem表示土壤温度,SM代表土壤含水量,Cond代表土壤电导率,NH4+-N代表土壤铵态氮含量。
Fig. 2 Redundancy analysis (RDA) analysis on AOA/AOB amoA gene abundance and selected environmental factors. Tem represents soil temperature, SM represents soil moisture, Cond represents soil conductivity, and NH4+-N represents soil ammonium nitrogen content.
| 差异来源 Source | 自由度 df | f值 f | Pr > F | |
|---|---|---|---|---|
| 氨氧化古菌 AOA amoA | 处理 Treatment | 1 | 0.13 | 0.725 |
| 土层 Layer | 3 | 64.00 | < 0.001 | |
| 处理 × 土层 Treatment × layer | 3 | 9.45 | < 0.001 | |
| 氨氧化细菌 AOB amoA | 处理 Treatment | 1 | 118.85 | < 0.001 |
| 土层 Layer | 3 | 128.34 | < 0.001 | |
| 处理 × 土层 Treatment × layer | 3 | 103.57 | < 0.001 | |
| 潜在硝化速率 PNR | 处理 Treatment | 1 | 24.03 | < 0.001 |
| 土层 Layer | 3 | 50.81 | < 0.001 | |
| 处理 × 土层 Treatment × layer | 3 | 8.69 | < 0.001 |
表2 AOA和AOB amoA基因丰度和土壤潜在硝化速率的方差分析
Table 2 Variance analyses of AOA/AOB amoA gene abundance and potential nitrification rate (PNR)
| 差异来源 Source | 自由度 df | f值 f | Pr > F | |
|---|---|---|---|---|
| 氨氧化古菌 AOA amoA | 处理 Treatment | 1 | 0.13 | 0.725 |
| 土层 Layer | 3 | 64.00 | < 0.001 | |
| 处理 × 土层 Treatment × layer | 3 | 9.45 | < 0.001 | |
| 氨氧化细菌 AOB amoA | 处理 Treatment | 1 | 118.85 | < 0.001 |
| 土层 Layer | 3 | 128.34 | < 0.001 | |
| 处理 × 土层 Treatment × layer | 3 | 103.57 | < 0.001 | |
| 潜在硝化速率 PNR | 处理 Treatment | 1 | 24.03 | < 0.001 |
| 土层 Layer | 3 | 50.81 | < 0.001 | |
| 处理 × 土层 Treatment × layer | 3 | 8.69 | < 0.001 |
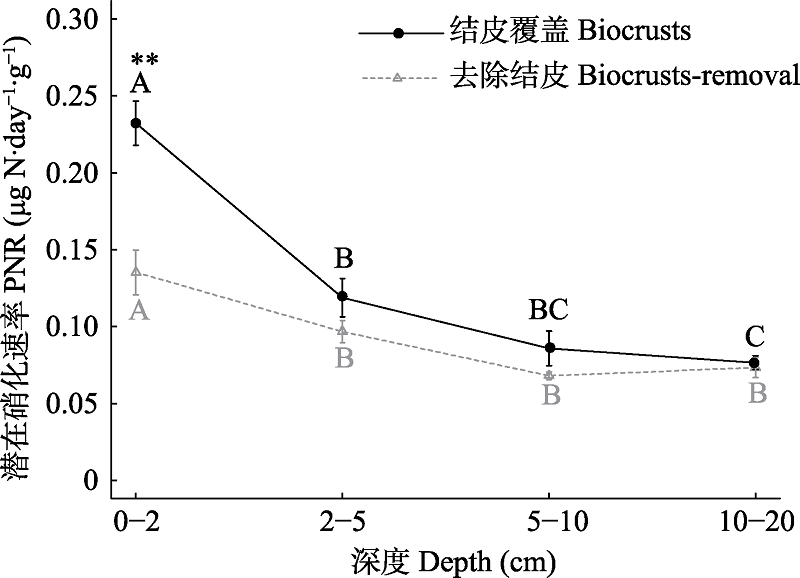
图3 结皮覆盖与去除结皮不同荒漠土层潜在硝化速率。大写字母表示相同结皮处理不同土层显著差异性,α = 0.05; *表示相同土层不同结皮处理之间差异性; * P < 0.05, ** P < 0.01。
Fig. 3 Potential nitrification rate (PNR) of different soil depths in biocrusts and biocrusts-removal soil. Capital letters indicate significant differences among soil depth in biocrusts or biocrusts-removal treatments, α = 0.05; * indicates the difference between biocrusts and biocrusts-removal treatments for the same soil depth; * P < 0.05, ** P < 0.01.
| 潜在硝化速率 Potential nitrification rate | ||
|---|---|---|
| 结皮覆盖土壤 Biocrusts soil | 去除结皮土壤 Biocrusts-removal soil | |
| 氨氧化古菌 AOA | ‒0.73** | ‒0.06 |
| 氨氧化细菌 AOB | 0.88** | 0.76** |
| 温度 Tem | 0.90** | 0.66* |
| 含水量 SM | 0.91** | 0.87** |
| pH | ‒0.79** | ‒0.44 |
| 电导率 Cond | 0.75** | 0.48 |
| 全碳 TC | 0.05 | 0.09 |
| 全氮 TN | 0.80** | ‒0.26 |
| 铵态氮 NH4+-N | 0.93** | 0.36 |
| 硝态氮 NO3--N | ‒0.60* | ‒0.08 |
表3 结皮覆盖土壤和去除结皮土壤的潜在硝化速率与AOA/AOB amoA基因丰度及环境参数相关性分析
Table 3 Pearson correlation analyses of potential nitrification rate (PNR) and AOA/AOB amoA gene abundance and environmental factors in Biocrusts and Biocrusts-removal soil
| 潜在硝化速率 Potential nitrification rate | ||
|---|---|---|
| 结皮覆盖土壤 Biocrusts soil | 去除结皮土壤 Biocrusts-removal soil | |
| 氨氧化古菌 AOA | ‒0.73** | ‒0.06 |
| 氨氧化细菌 AOB | 0.88** | 0.76** |
| 温度 Tem | 0.90** | 0.66* |
| 含水量 SM | 0.91** | 0.87** |
| pH | ‒0.79** | ‒0.44 |
| 电导率 Cond | 0.75** | 0.48 |
| 全碳 TC | 0.05 | 0.09 |
| 全氮 TN | 0.80** | ‒0.26 |
| 铵态氮 NH4+-N | 0.93** | 0.36 |
| 硝态氮 NO3--N | ‒0.60* | ‒0.08 |
| [1] |
Adair KL, Schwartz E (2008) Evidence that ammonia-oxidizing Archaea are more abundant than ammonia-oxidizing bacteria in semiarid soils of northern Arizona, USA. Microbial Ecology, 56, 420-426.
URL PMID |
| [2] | Belnap J (1996) Soil surface disturbances in cold deserts: Effects on nitrogenase activity in cyanobacterial-lichen soil crusts. Biology and Fertility of Soils, 23, 362-367. |
| [3] | Belnap J (2002) Impacts of off-road vehicles on nitrogen cycles in biological soil crusts: Resistance in different U. S. deserts. Journal of Arid Environments, 52, 155-165. |
| [4] | Belnap J (2003) The world at your feet: Desert biological soil crusts. Frontiers in Ecology and the Environment, 1, 181-189. |
| [5] |
Belnap J, Phillips SL, Miller ME (2004) Response of desert biological soil crusts to alterations in precipitation frequency. Oecologia, 141, 306-316.
URL PMID |
| [6] | Condon LA, Pyke DA (2018) Resiliency of biological soil crusts and vascular plants varies among morphogroups with disturbance intensity. Plant and Soil, 433, 271-287. |
| [7] | Delgado-Baquerizo M, Maestre FT, Eldridge DJ, Singh BK (2016) Microsite differentiation drives the abundance of soil ammonia oxidizing bacteria along aridity gradients. Frontiers in Microbiology, 7, 505. |
| [8] |
Di HJ, Cameron KC, Shen JP, Winefield CS, O’Callaghan M, Bowatte S, He JZ (2010) Ammonia-oxidizing bacteria and archaea grow under contrasting soil nitrogen conditions. FEMS Microbiology Ecology, 72, 386-394.
URL PMID |
| [9] | Eldridge DJ, Freudenberger D, Koen TB (2006) Diversity and abundance of biological soil crust taxa in relation to fine and coarse-scale disturbances in a grassy eucalypt woodland in Eastern Australia. Plant and Soil, 281, 255-268. |
| [10] | Eldridge DJ, Soliveres S, Bowker MA, Val J (2013) Grazing dampens the positive effects of shrub encroachment on ecosystem functions in a semi-arid woodland. Journal of Applied Ecology, 50, 1028-1038. |
| [11] | Erguder TH, Boon N, Wittebolle L, Marzorati M, Verstraete W (2009) Environmental factors shaping the ecological niches of ammonia-oxidizing archaea. FEMS Microbiology Reviews, 33, 855-869. |
| [12] | Ferrenberg S, Reed SC, Belnap J (2015) Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proceedings of the National Academy of Sciences, USA, 112, 12116-12121. |
| [13] | Ferrenberg S, Tucker CL, Reed SC (2017) Biological soil crusts: Diminutive communities of potential global importance. Frontiers in Ecology and the Environment, 15, 160-167. |
| [14] | Francis CA, Roberts KJ, Beman JM, Santoro AE, Oakley BB (2005) Ubiquity and diversity of ammonia-oxidizing archaea in water columns and sediments of the ocean. Proceedings of the National Academy of Sciences, USA, 102, 14683-14688. |
| [15] | Garcia V, Aranibar J, Pietrasiak N (2015) Multiscale effects on biological soil crusts cover and spatial distribution in the Monte Desert. Acta Oecologica, 69, 35-45. |
| [16] |
Garcia-Pichel F, Johnson SL, Youngkin D, Belnap J (2003) Small-scale vertical distribution of bacterial biomass and diversity in biological soil crusts from arid lands in the Colorado Plateau. Microbial Ecology, 46, 312-321.
URL PMID |
| [17] | Hu BL, Liu S, Wang W, Shen LD, Lou LP, Liu WP, Tian GM, Xu XY, Zheng P (2014) pH-dominated niche segregation of ammonia-oxidising microorganisms in Chinese agricultural soils. FEMS Microbiology Ecology, 90, 290-299. |
| [18] | Hu R, Wang XP, Pan YX, Zhang YF, Zhang H, Chen N (2015) Seasonal variation of net N mineralization under different biological soil crusts in Tengger Desert, North China. CATENA, 127, 9-16. |
| [19] | Johnson SL, Budinoff CR, Belnap J, Garcia-Pichel F (2005) Relevance of ammonium oxidation within biological soil crust communities. Environmental Microbiology, 7, 1-12. |
| [20] | Johnson SL, Neuer S, Garcia-Pichel F (2007) Export of nitrogenous compounds due to incomplete cycling within biological soil crusts of arid lands. Environmental Microbiology, 9, 680-689. |
| [21] |
Coe KK, Belnap J, Sparks JP (2012) Precipitation-driven carbon balance controls survivorship of desert biocrust mosses. Ecology, 93, 1626-1636.
DOI URL PMID |
| [22] |
Kowalchuk GA, Stephen JR (2001) Ammonia-oxidizing bacteria: A model for molecular microbial ecology. Annual Review of Microbiology, 55, 485-529.
URL PMID |
| [23] |
Kurola J, Salkinoja-Salonen M, Aarnio T, Hultman J, Romantschuk M (2005) Activity, diversity and population size of ammonia-oxidising bacteria in oil-contaminated landfarming soil. FEMS Microbiology Letters, 250, 33-38.
DOI URL PMID |
| [24] |
Leininger S, Urich T, Schloter M, Schwark L, Qi J, Nicol GW, Prosser JI, Schuster SC, Schleper C (2006) Archaea predominate among ammonia-oxidizing prokaryotes in soils. Nature, 442, 806-809.
URL PMID |
| [25] |
Liu JJ, Yu ZH, Yao Q, Sui YY, Shi Y, Chu HY, Tang CX, Franks AE, Jin J, Liu XB, Wang GH (2018) Ammonia-oxidizing archaea show more distinct biogeographic distribution patterns than ammonia-oxidizing bacteria across the black soil zone of Northeast China. Frontiers in Microbiology, 9, 171.
URL PMID |
| [26] | Liu RY, Li K, Zhang HX, Zhu JG, Joshi D (2014) Spatial distribution of microbial communities associated with dune landform in the Gurbantunggut Desert, China. Journal of Microbiology, 52, 898-907. |
| [27] |
Magalhães CM, Machado A, Frank-Fahle B, Lee CK, Cary SC (2014) The ecological dichotomy of ammonia-oxidizing archaea and bacteria in the hyper-arid soils of the Antarctic Dry Valleys. Frontiers in Microbiology, 5, 515.
DOI URL PMID |
| [28] | Marusenko Y, Bates ST, Anderson I, Johnson SL, Soule TY, Garcia-Pichel F (2013) Ammonia-oxidizing archaea and bacteria are structured by geography in biological soil crusts across North American arid lands. Ecological Processes, 2, 1-10. |
| [29] | Matzner E, Borken W (2008) Do freeze-thaw events enhance C and N losses from soils of different ecosystems? A review. European Journal of Soil Science, 59, 274-284. |
| [30] |
Prosser JI, Nicol GW (2012) Archaeal and bacterial ammonia-oxidisers in soil: The quest for niche specialisation and differentiation. Trends in Microbiology, 20, 523-531.
DOI URL PMID |
| [31] |
Rotthauwe JH, Witzel KP, Liesack W (1997) The ammonia monooxygenase structural gene amoA as a functional marker: Molecular fine-scale analysis of natural ammonia-oxidizing populations. Applied and Environmental Microbiology, 63, 4704-4712.
DOI URL PMID |
| [32] | Ferrenberg S, Reed SC, Belnap J (2015) Climate change and physical disturbance cause similar community shifts in biological soil crusts. Proceedings of the National Academy of Sciences, USA, 112, 12116-12121. |
| [33] | Steinweg JM, Fisk MC, McAlexander B, Groffman PM, Hardy JP (2008) Experimental snowpack reduction alters organic matter and net N mineralization potential of soil macroaggregates in a northern hardwood forest. Biology and Fertility of Soils, 45, 1-10. |
| [34] |
Stempfhuber B, Engel M, Fischer D, Neskovic-Prit G, Wubet T, Schöning I, Gubry-Rangin C, Kublik S, Schloter-Hai B, Rattei T, Welzl G, Nicol GW, Schrumpf M, Buscot F, Prosser JI, Schloter M (2015) pH as a driver for ammonia-oxidizing archaea in forest soils. Microbial Ecology, 69, 879-883.
URL PMID |
| [35] | Tao JJ, Bai TS, Xiao R, Wang P, Wang FW, Duryee AM, Wang Y, Zhang Y, Hu SJ (2018) Vertical distribution of ammonia-oxidizing microorganisms across a soil profile of the Chinese Loess Plateau and their responses to nitrogen inputs. Science of The Total Environment, 635, 240-248. |
| [36] |
Valentine DL (2007) Adaptations to energy stress dictate the ecology and evolution of the Archaea. Nature Reviews Microbiology, 5, 316-323.
URL PMID |
| [37] |
Verhamme DT, Prosser JI, Nicol GW (2011) Ammonia concentration determines differential growth of ammonia-oxidising archaea and bacteria in soil microcosms. The ISME Journal, 5, 1067-1071.
URL PMID |
| [38] | Weber B, Wu DM, Tamm A, Ruckteschler N, Rodríguez-Caballero E, Steinkamp J, Meusel H, Elbert W, Behrendt T, Sörgel M, Cheng YF, Crutzen PJ, Su H, Pöschl U (2015) Biological soil crusts accelerate the nitrogen cycle through large NO and HONO emissions in drylands. Proceedings of the National Academy of Sciences, USA, 112, 15384-15389. |
| [39] | Wu L, Zhang YM, Zhang J, Downing A (2015) Precipitation intensity is the primary driver of moss crust-derived CO2 exchange: Implications for soil C balance in a temperate desert of northwestern China. European Journal of Soil Biology, 67, 27-34. |
| [40] | Wu N, Zhang YM, Downing A (2009) Comparative study of nitrogenase activity in different types of biological soil crusts in the Gurbantunggut Desert, Northwestern China. Journal of Arid Environments, 73, 828-833. |
| [41] | Xiao R, Qiu YP, Tao JJ, Zhang XL, Chen HH, Reberg-Horton SC, Shi W, Shew HD, Zhang Y, Hu SJ (2019) Biological controls over the abundances of terrestrial ammonia oxidizers. Global Ecology and Biogeography, 29, 384-399. |
| [42] |
Yao HY, Campbell CD, Chapman SJ, Freitag TE, Nicol GW, Singh BK (2013) Multi-factorial drivers of ammonia oxidizer communities: Evidence from a national soil survey. Environmental Microbiology, 15, 2545-2556.
DOI URL PMID |
| [43] | Zhang BC, Zhang YM, Su YG, Wang JZ, Zhang J (2013) Responses of microalgal-microbial biomass and enzyme activities of biological soil crusts to moisture and inoculated microcoleus vaginatus gradients. Arid Land Research and Management, 27, 216-230. |
| [44] | Zhang CP, Niu DC, Song ML, Elser JJ, Okie JG, Fu H (2018) Effects of rainfall manipulations on carbon exchange of cyanobacteria and moss-dominated biological soil crusts. Soil Biology and Biochemistry, 124, 24-31. |
| [45] | Zhang YM, Chen J, Wang L, Wang XQ, Gu ZH (2007) The spatial distribution patterns of biological soil crusts in the Gurbantunggut Desert, Northern Xinjiang, China. Journal of Arid Environments, 68, 599-610. |
| [46] | Zhao RM, Hui R, Liu LC, Xie M, An LZ (2018) Effects of snowfall depth on soil physical-chemical properties and soil microbial biomass in moss-dominated crusts in the Gurbantunggut Desert, Northern China. CATENA, 169, 175-182. |
| [47] |
Zhou XB, Smith H, Giraldo SA, Belnap J, Garcia-Pichel F (2016) Differential responses of dinitrogen fixation, diazotrophic cyanobacteria and ammonia oxidation reveal a potential warming-induced imbalance of the N-cycle in biological soil crusts. PLoS ONE, 11, e0164932.
DOI URL PMID |
| [48] | Zhou XB, Tao Y, Yin BF, Tucker C, Zhang YM (2020) Nitrogen pools in soil covered by biological soil crusts of different successional stages in a temperate desert in Central Asia. Geoderma, 366, 114166. |
| [49] | Zhou XB, Zhang YM (2014a) Season and nitrogen effects on activities of three hydrolytic enzymes in soils of the gurbantunggut desert, northwest China. Communications in Soil Science and Plant Analysis, 45, 1699-1713. |
| [50] | Zhou XB, Zhang YM (2014b) Seasonal pattern of soil respiration and gradual changing effects of nitrogen addition in a soil of the Gurbantunggut Desert, northwestern China. Atmospheric Environment, 85, 187-194. |
| [51] | Zhou XQ, Chen CR, Wang YF, Xu ZH, Duan JC, Hao YB, Smaill S (2013) Soil extractable carbon and nitrogen, microbial biomass and microbial metabolic activity in response to warming and increased precipitation in a semiarid Inner Mongolian grassland. Geoderma, 206, 24-31. |
| [52] | Zhuang WW, Downing A, Zhang YM (2015) The influence of biological soil crusts on 15N translocation in soil and vascular plant in a temperate desert of northwestern China . Journal of Plant Ecology, 8, 420-428. |
| [1] | 陈静, 张丙昌, 刘燕晋, 武杰, 赵康, 明姣. 荒漠生物结皮细鞘丝藻类(Leptolyngbya-like)蓝藻多样性[J]. 生物多样性, 2024, 32(9): 24186-. |
| [2] | 张雅丽, 张丙昌, 赵康, 李凯凯, 刘燕晋. 毛乌素沙地不同类型生物结皮细菌群落差异及其驱动因子[J]. 生物多样性, 2023, 31(8): 23027-. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn
![]()