



生物多样性 ›› 2024, Vol. 32 ›› Issue (3): 23384. DOI: 10.17520/biods.2023384 cstr: 32101.14.biods.2023384
收稿日期:2023-10-15
接受日期:2024-01-10
出版日期:2024-03-20
发布日期:2024-01-12
通讯作者:
*E-mail: wylongceltics@163.com
基金资助:
Zhang Xuan, Xu Ying, Yang Yanci, Zhao Yanling, Men Zhonghua, Wang Yonglong*( )
)
Received:2023-10-15
Accepted:2024-01-10
Online:2024-03-20
Published:2024-01-12
Contact:
*E-mail: wylongceltics@163.com
摘要:
叶际微生物在维持植物健康生长方面具有重要的作用, 然而, 目前我们对珍稀孑遗植物半日花(Helianthemum songaricum)叶际真菌的多样性和群落结构等知之甚少。因此, 本研究利用Illumina高通量测序技术检测西鄂尔多斯自然保护区半日花叶片表生和内生真菌的多样性, 探究其网络结构特征以及群落构建的机制。结果显示: 棋盘井地区的叶际表生真菌丰富度指数(156.38 ± 8.42)显著高于内生真菌(111.13 ± 5.57), 棋盘井叶际表生真菌丰富度指数显著高于拉僧庙(125.57 ± 7.20)和千里山(114.75 ± 10.35), 而拉僧庙的内生真菌丰富度指数(155.71 ± 15.40)显著高于棋盘井。叶际真菌以子囊菌门、毛霉菌门和担子菌门为优势门, 叶际表生真菌和内生真菌分别在3个不同地点具有显著的指示类群, 叶片部位(即叶表和叶内)和地点显著影响叶片真菌的群落组成。共存网络分析表明, 叶际表生和内生真菌主要是协同作用, 拮抗作用较小。半日花叶际表生和内生真菌群落的构建主要由随机性过程驱动。综上所述, 半日花叶际表生和内生真菌的多样性和群落组成受到叶片部位(叶表和叶内)和地点的显著影响, 随机性过程主导叶际表生和内生真菌的群落构建。研究结果可为珍稀濒危植物的保护和合理利用提供一定的科学基础和实践指导。
张旋, 徐颖, 杨颜慈, 赵艳玲, 门中华, 王永龙 (2024) 孑遗植物半日花叶际真菌群落的多样性与构建机制. 生物多样性, 32, 23384. DOI: 10.17520/biods.2023384.
Zhang Xuan, Xu Ying, Yang Yanci, Zhao Yanling, Men Zhonghua, Wang Yonglong (2024) The diversity and assembly mechanism of phyllosphere fungal communities in the relict plant Helianthemum songaricum. Biodiversity Science, 32, 23384. DOI: 10.17520/biods.2023384.
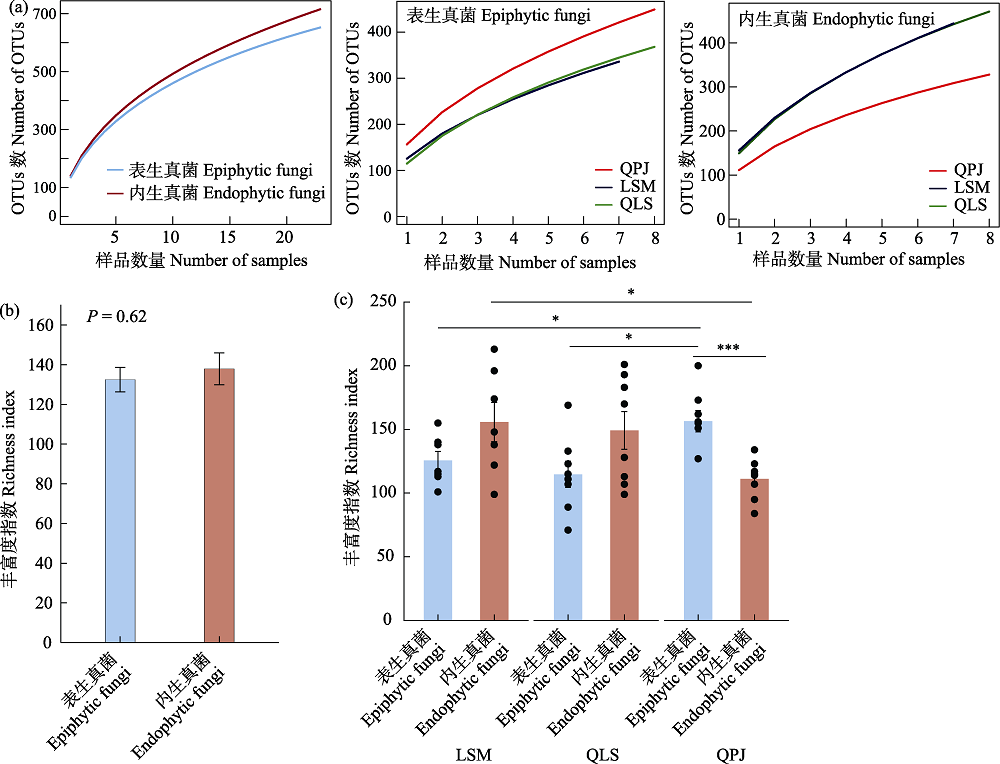
图1 半日花叶际真菌多样性。(a)物种累积曲线; (b)叶际表生和内生真菌的丰富度指数; (c)叶际表生和内生真菌不同采样地点丰富度指数。LSM: 拉僧庙; QLS: 千里山; QPJ: 棋盘井。误差线表示标准误差。
Fig. 1 Diversity of phyllosphere fungi in Helianthemum songaricum. (a) Species accumulation curves; (b) Richness index of phyllosphere epiphytic and endophytic fungi; (c) Richness indices for different sampling locations of phyllosphere epiphytic and endophytic fungi. LSM, Lasengmiao; QLS, Qianlishan; QPJ, Qipanjing. * P < 0.05; *** P < 0.001.
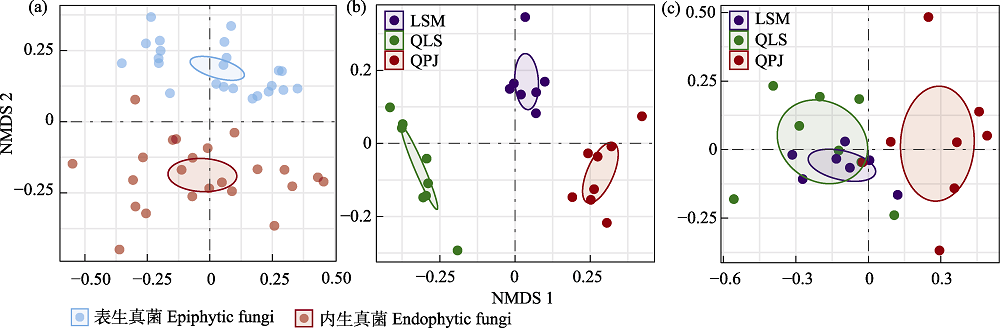
图2 基于Bray-Curtis距离的半日花叶际真菌群落非度量多维尺度排序(NMDS)分析。(a)叶际表生和内生真菌总体; (b)叶际表生真菌; (c)叶际内生真菌。LSM: 拉僧庙; QLS: 千里山; QPJ: 棋盘井。
Fig. 2 Nonmetric multidimensional scaling (NMDS) analysis of phyllosphere fungal communities in Helianthemum songaricum based on Bray-Curtis distance. (a) Overall phyllosphere epiphytic and endophytic fungi; (b) Phyllosphere epiphytic fungi; (c) Phyllosphere endophytic fungi. LSM, Lasengmiao; QLS, Qianlishan; QPJ, Qipanjing.
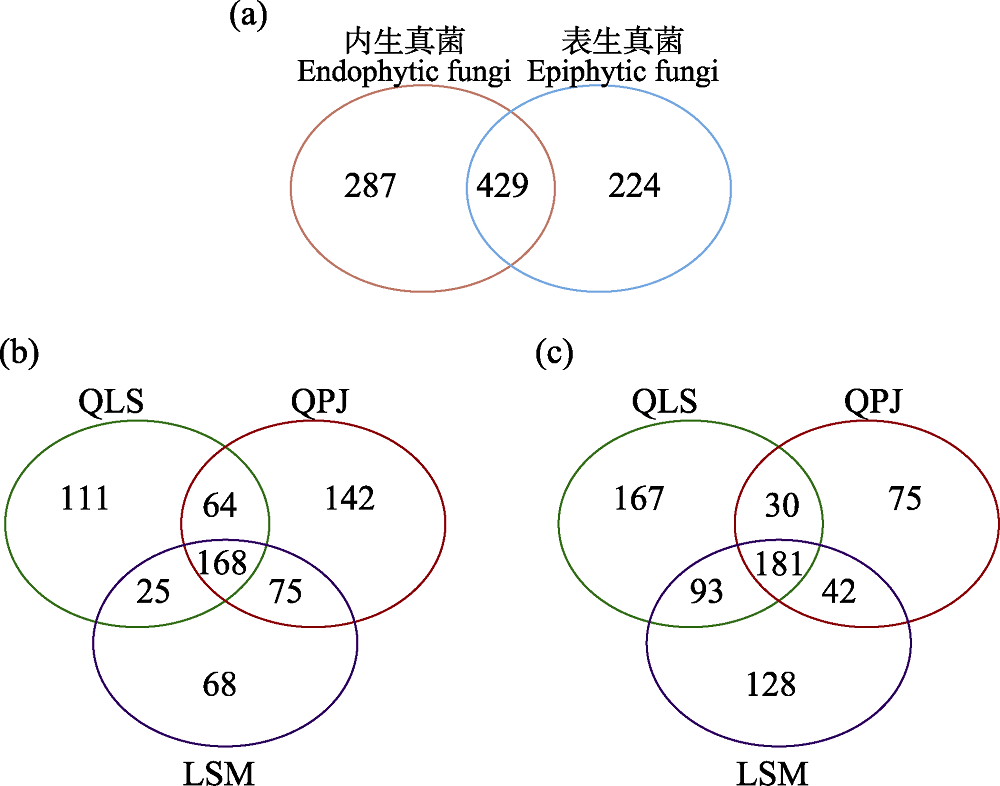
图3 半日花叶际真菌OTUs分布Venn图。(a)叶际表生和内生真菌总体; (b)叶际表生真菌; (c)叶际内生真菌。LSM: 拉僧庙; QLS: 千里山; QPJ: 棋盘井。
Fig. 3 Venn diagram depicting the distribution of phyllosphere fungal OTUs in Helianthemum songaricum. (a) Overall phyllosphere epiphytic and endophytic fungi; (b) Phyllosphere epiphytic fungi; (c) Phyllosphere endophytic fungi. LSM, Lasengmiao; QLS, Qianlishan; QPJ, Qipanjing.
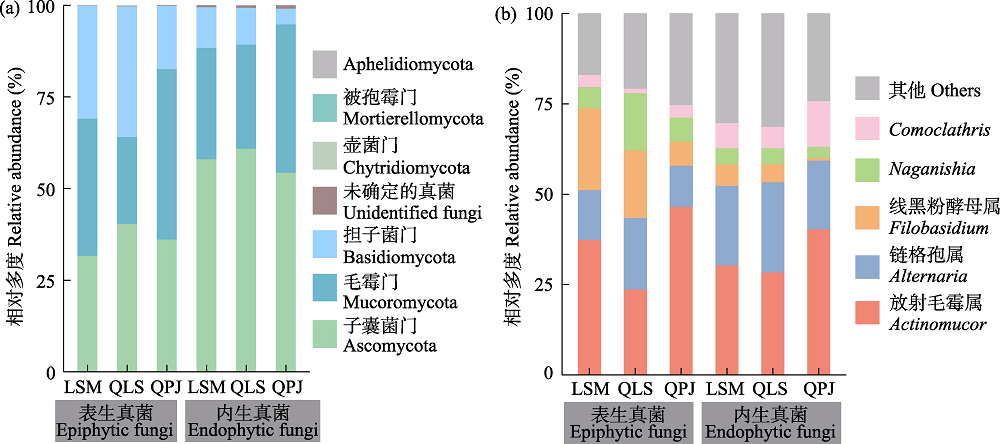
图4 半日花叶际真菌群落门和属水平的相对多度。(a)门水平; (b)属水平。LSM: 拉僧庙; QLS: 千里山; QPJ: 棋盘井。叶际表生和内生真菌总序列数相对多度< 5.00%的属及未鉴定属归于“其他”。
Fig. 4 Relative abundance of phyllosphere fungal communities at the phylum and genus levels in Helianthemum songaricum. (a) Phylum level; (b) Genus level. LSM, Lasengmiao; QLS, Qianlishan; QPJ, Qipanjing. Genera with a relative abundance of < 5.00% of the total number of sequences of phyllosphere epiphytic and endophytic fungi and unidentified genera were assigned to “Others”.
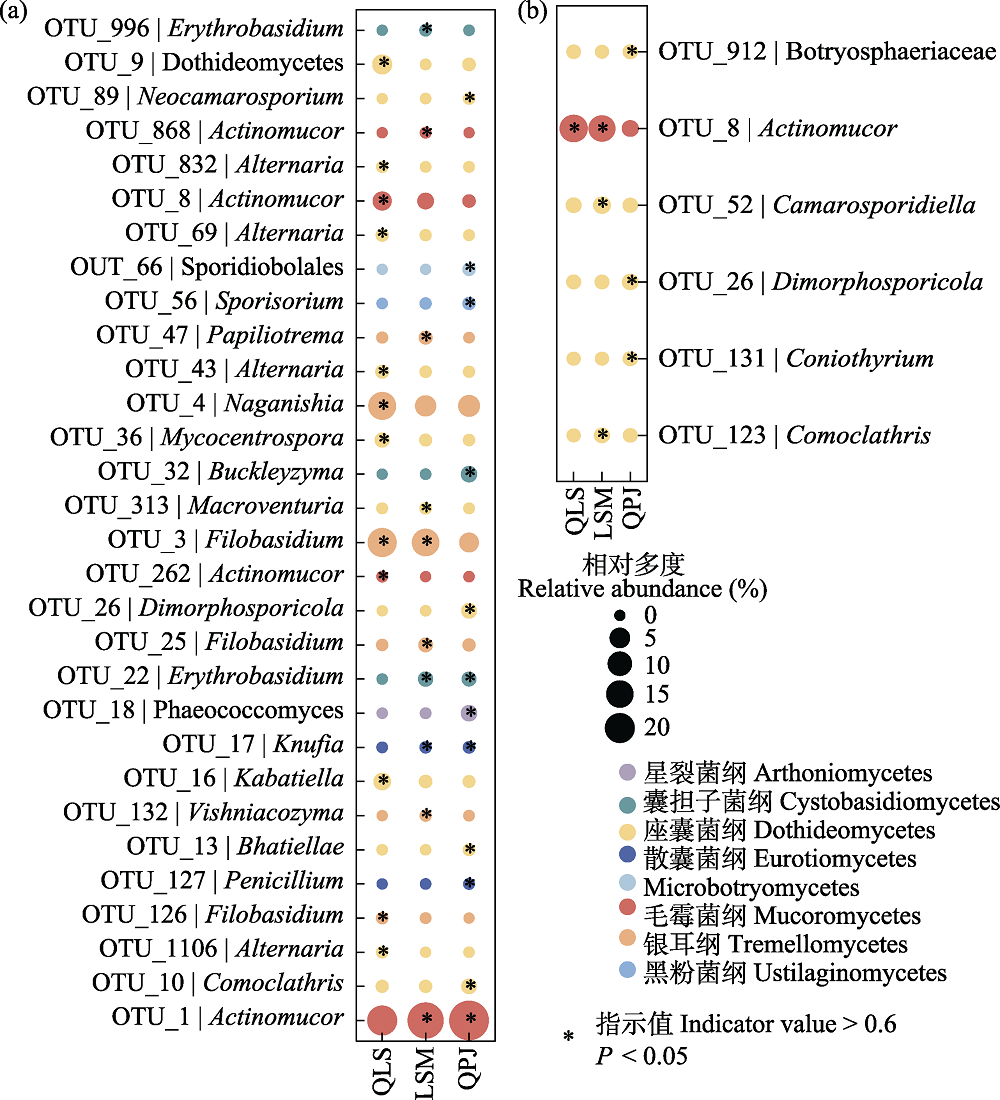
图5 半日花叶际真菌指示种分析。(a)叶际表生真菌; (b)叶际内生真菌。LSM: 拉僧庙; QLS: 千里山; QPJ: 棋盘井。
Fig. 5 Indicator species analysis of phyllosphere fungi in Helianthemum songaricum. (a) Phyllosphere epiphytic fungi; (b) Phyllosphere endophytic fungi. LSM, Lasengmiao; QLS, Qianlishan; QPJ, Qipanjing.
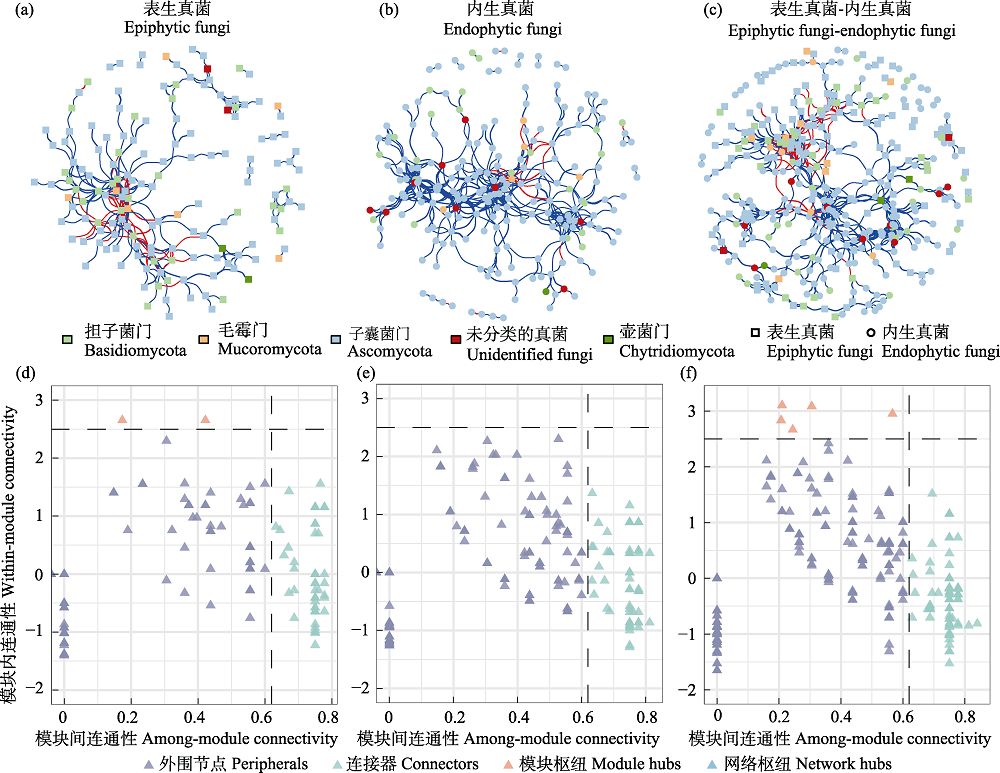
图6 半日花叶际真菌的共存网络分析。(a)表生真菌; (b)内生真菌; (c)表生真菌-内生真菌; (d)、(e)和(f)分别表示表生真菌、内生真菌、表生真菌-内生真菌的模块内连通性(Zi)和模块间连通性(Pi)。
Fig. 6 Co-occurrence network analysis of phyllosphere fungi in Helianthemum songaricum. (a) Epiphytic fungi; (b) Endophytic fungi; (c) Epiphytic fungi-endophytic fungi. (d), (e), and (f) represent within-module connectivity (Zi) and among-module connectivity (Pi) for epiphytic fungi, endophytic fungi, and epiphytic fungi-endophytic fungi, respectively.
| 表生真菌 Epiphytic fungi | 内生真菌Endophytic fungi | 表生真菌-内生真菌 Epiphytic fungi-endophytic fungi | |
|---|---|---|---|
| 总节点 Total nodes | 153 | 214 | 357 |
| 总边 Total edges | 282 | 481 | 695 |
| 平均度 Average degree | 3.686 | 4.495 | 3.894 |
| 平均路径长度 Average path length | 5.966 | 4.936 | 7.149 |
| 平均聚集系数 Average clustering coefficient | 0.419 | 0.381 | 0.430 |
| 连接性 Connectance | 0.024 | 0.021 | 0.011 |
| 负相关边比例 Negative edges percentage (%) | 21.99 | 4.78 | 12.52 |
| 模块化指数 Modularity | 0.677 | 0.66 | 0.799 |
表1 半日花叶际真菌网络拓扑参数
Table 1 Topological parameters of the phyllosphere fungal network of Helianthemum songaricum
| 表生真菌 Epiphytic fungi | 内生真菌Endophytic fungi | 表生真菌-内生真菌 Epiphytic fungi-endophytic fungi | |
|---|---|---|---|
| 总节点 Total nodes | 153 | 214 | 357 |
| 总边 Total edges | 282 | 481 | 695 |
| 平均度 Average degree | 3.686 | 4.495 | 3.894 |
| 平均路径长度 Average path length | 5.966 | 4.936 | 7.149 |
| 平均聚集系数 Average clustering coefficient | 0.419 | 0.381 | 0.430 |
| 连接性 Connectance | 0.024 | 0.021 | 0.011 |
| 负相关边比例 Negative edges percentage (%) | 21.99 | 4.78 | 12.52 |
| 模块化指数 Modularity | 0.677 | 0.66 | 0.799 |
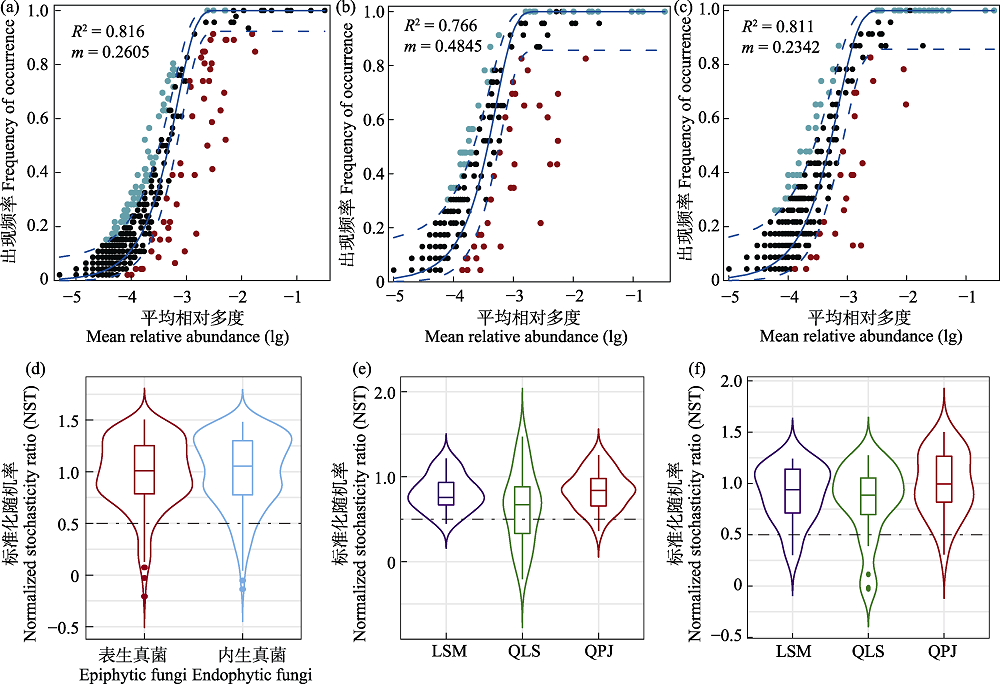
图7 半日花叶际真菌群落的生态构建机制。(a)、(b)和(c)分别表示叶际总真菌、表生真菌以及内生真菌群落的中性群落模型分析; (d)叶际表生和内生真菌标准化随机率(NST)分析、(e)表生真菌NST分析; (f)内生真菌NST分析。LSM: 拉僧庙; QLS: 千里山; QPJ: 棋盘井。
Fig. 7 Ecological assembly mechanisms of phyllosphere fungal communities in Helianthemum songaricum. (a), (b) and (c) represent neutral community model analyses for all ecological niches, phyllosphere epiphytic fungi, and phyllosphere endophytic fungi, respectively. (d) Normalized stochastic rate (NST) analyses for phyllosphere epiphytic and endophytic fungi. (e) NST analyses for phyllosphere epiphytic fungi. (f) NST analyses for endophytic fungi. LSM, Lasengmiao; QLS, Qianlishan; QPJ, Qipanjing.
| [1] |
Arnold AE, Henk DA, Eells RL, Lutzoni F, Vilgalys R (2007) Diversity and phylogenetic affinities of foliar fungal endophytes in loblolly pine inferred by culturing and environmental PCR. Mycologia, 99, 185-206.
PMID |
| [2] | Arnold AE, Mejía LC, Kyllo D, Rojas EI, Maynard Z, Robbins N, Herre EA (2003) Fungal endophytes limit pathogen damage in a tropical tree. Proceedings of the National Academy of Sciences, USA, 100, 15649-15654. |
| [3] | Barberán A, Bates ST, Casamayor EO, Fierer N (2012) Using network analysis to explore co-occurrence patterns in soil microbial communities. The ISME Journal, 6, 343-351. |
| [4] | Bashir I, War AF, Rafiq I, Reshi ZA, Rashid I, Shouche YS (2022) Phyllosphere microbiome: Diversity and functions. Microbiological Research, 254, 126888. |
| [5] | Carlström CI, Field CM, Bortfeld-Miller M, Müller B, Sunagawa S, Vorholt JA (2019) Synthetic microbiota reveal priority effects and keystone strains in the Arabidopsis phyllosphere. Nature Ecology & Evolution, 3, 1445-1454. |
| [6] | Chen SY, Zhao XY, Li GF, Liu T, Ren PJ, Shi S, Feng JC (2023) Physiological and ecological responses of Helianthemum songoricum under different water conditions. Journal of Minzu University of China (Natural Sciences Edition), 32(2), 10-16. (in Chinese with English abstract) |
| [ 陈思瑜, 赵心雨, 李国芳, 刘彤, 任珮君, 石莎, 冯金朝 (2023) 不同水分条件下半日花的生理生态响应. 中央民族大学学报(自然科学版), 32(2), 10-16.] | |
| [7] | Chen Y (2014) The Study of Community Ecology on Helianthemum songoricum. PhD dissertation, Inner Mongolia University, Hohhot. (in Chinese with English abstract) |
| [ 陈育 (2014) 西鄂尔多斯半日花群落生态学研究. 博士学位论文, 内蒙古大学, 呼和浩特.] | |
| [8] |
Coleman-Derr D, Desgarennes D, Fonseca-Garcia C, Gross S, Clingenpeel S, Woyke T, North G, Visel A, Partida-Martinez LP, Tringe SG (2016) Plant compartment and biogeography affect microbiome composition in cultivated and native Agave species. New Phytologist, 209, 798-811.
DOI PMID |
| [9] | Cui Z, Zhang X, Yang H, Sun L (2017) Bioremediation of heavy metal pollution utilizing composite microbial agent of Mucor circinelloides, Actinomucor sp. and Mortierella sp. Journal of Environmental Chemical Engineering, 5, 3616-3621. |
| [10] |
Dini-Andreote F, Raaijmakers JM (2018) Embracing community ecology in plant microbiome research. Trends in Plant Science, 23, 467-469.
DOI PMID |
| [11] | Fang SB, Zhang XS (2013) Control of vegetation distribution: Climate, geological substrate, and geomorphic factors. A case study of grassland in Ordos, Inner Mongolia, China. Canadian Journal of Remote Sensing, 39, 167-174. |
| [12] |
Fitzpatrick CR, Salas-González I, Conway JM, Finkel OM, Gilbert S, Russ D, Teixeira PJPL, Dangl JL (2020) The plant microbiome: From ecology to reductionism and beyond. Annual Review of Microbiology, 74, 81-100.
DOI PMID |
| [13] |
Fonseca-García C, Coleman-Derr D, Garrido E, Visel A, Tringe SG, Partida-Martínez LP (2016) The cacti microbiome: Interplay between habitat-filtering and host-specificity. Frontiers in Microbiology, 7, 150.
DOI PMID |
| [14] |
Gao C, Xu L, Montoya L, Madera M, Hollingsworth J, Chen L, Purdom E, Singan V, Vogel J, Hutmacher RB, Dahlberg JA, Coleman-Derr D, Lemaux PG, Taylor JW (2022) Co-occurrence networks reveal more complexity than community composition in resistance and resilience of microbial communities. Nature Communications, 13, 3867.
DOI PMID |
| [15] |
Gomes T, Pereira JA, Benhadi J, Lino-Neto T, Baptista P (2018) Endophytic and epiphytic phyllosphere fungal communities are shaped by different environmental factors in a Mediterranean ecosystem. Microbial Ecology, 76, 668-679.
DOI PMID |
| [16] | Gourion B, Rossignol M, Vorholt JA (2006) A proteomic study of Methylobacterium extorquens reveals a response regulator essential for epiphytic growth. Proceedings of the National Academy of Sciences, USA, 103, 13186-13191. |
| [17] | Guerreiro MA, Brachmann A, Begerow D, Peršoh D (2018) Transient leaf endophytes are the most active fungi in 1-year-old beech leaf litter. Fungal Diversity, 89, 237-251. |
| [18] |
Guo LD, Hyde KD, Liew ECY (2000) Identification of endophytic fungi from Livistona chinensis based on morphology and rDNA sequences. New Phytologist, 147, 617-630.
DOI PMID |
| [19] | Huang SL, Zha XJ, Fu G (2023) Affecting factors of plant phyllosphere microbial community and their responses to climatic warming—A review. Plants, 12, 2891. |
| [20] | Jia T, Wang RH, Fan XH, Chai BF (2018) A comparative study of fungal community structure, diversity and richness between the soil and the phyllosphere of native grass species in a copper tailings dam in Shanxi Province, China. Applied Sciences, 8, 1297. |
| [21] |
Jiao S, Lu Y (2020) Soil pH and temperature regulate assembly processes of abundant and rare bacterial communities in agricultural ecosystems. Environmental Microbiology, 22, 1052-1065.
DOI PMID |
| [22] |
Jumpponen A, Jones KL (2009) Massively parallel 454 sequencing indicates hyperdiverse fungal communities in temperate Quercus macrocarpa phyllosphere. New Phytologist, 184, 438-448.
DOI PMID |
| [23] | Kadioglu A, Terzi R (2007) A dehydration avoidance mechanism: Leaf rolling. The Botanical Review, 73, 290-302. |
| [24] | Koskella B (2020) The phyllosphere. Current Biology, 30, R1143-R1146. |
| [25] | Larkin BG, Hunt LS, Ramsey PW (2012) Foliar nutrients shape fungal endophyte communities in western white pine (Pinus monticola) with implications for white-tailed deer herbivory. Fungal Ecology, 5, 252-260. |
| [26] | Li S, Wang H, Gou W, White JF, Kingsley KL, Wu G, Su P (2021) Leaf functional traits of dominant desert plants in the Hexi Corridor, northwestern China: Trade-off relationships and adversity strategies. Global Ecology and Conservation, 28, e01666. |
| [27] | Li ZJ, Sha N, Shi YB, Tong XZ, Dong L, Zhang XQ, Sun Q, Liang CZ (2019) Classification and characteristics of Helianthemum songaricum communities in western Erdos Region, Nei Mongol, China. Chinese Journal of Plant Ecology, 43, 806-816. (in Chinese with English abstract) |
|
[ 李紫晶, 莎娜, 史亚博, 佟旭泽, 董雷, 张小青, 孙蔷, 梁存柱 (2019) 内蒙古西鄂尔多斯地区半日花荒漠群落特征及其分类. 植物生态学报, 43, 806-816.]
DOI |
|
| [28] | Liu H, Li J, Carvalhais LC, Percy CD, Prakash Verma J, Schenk PM, Singh BK (2021) Evidence for the plant recruitment of beneficial microbes to suppress soil-borne pathogens. New Phytologist, 229, 2873-2885. |
| [29] | Liu Z, Wang C, Yang X, Liu G, Cui Q, Indree T, Ye X, Huang Z (2023) The relationship and influencing factors between endangered plant Tetraena mongolica and soil microorganisms in West Ordos desert ecosystem, northern China. Plants, 12, 1048. |
| [30] |
Melotto M, Underwood W, He SY (2008) Role of stomata in plant innate immunity and foliar bacterial diseases. Annual Review of Phytopathology, 46, 101-122.
DOI PMID |
| [31] | Mercado-Blanco J (2015) Life of microbes inside the plant. In: Principles of Plant-Microbe Interactions: Microbes for Sustainable Agriculture (ed. Lugtenberg B), pp. 25-32. Springer International Publishing, Cham. |
| [32] | Ning D, Deng Y, Tiedje JM, Zhou J (2019) A general framework for quantitatively assessing ecological stochasticity. Proceedings of the National Academy of Sciences, USA, 116, 16892-16898. |
| [33] | Osono T (2007) Endophytic and epiphytic phyllosphere fungi of red-osier dogwood (Cornus stolonifera) in British Columbia. Mycoscience, 48, 47-52. |
| [34] |
Paranjape K, Bédard É, Shetty D, Hu MQ, Choon FCP, Prévost M, Faucher SP (2020) Unravelling the importance of the eukaryotic and bacterial communities and their relationship with Legionella spp. ecology in cooling towers: A complex network. Microbiome, 8, 157.
DOI PMID |
| [35] | Purahong W, Hyde KD (2011) Effects of fungal endophytes on grass and non-grass litter decomposition rates. Fungal Diversity, 47, 1-7. |
| [36] | Qiu LP, Zhang Q, Zhu HS, Reich PB, Banerjee S, van der Heijden MGA, Sadowsky MJ, Ishii S, Jia XX, Shao MG, Liu BY, Jiao H, Li HQ, Wei XR (2021) Erosion reduces soil microbial diversity, network complexity and multi- functionality. The ISME Journal, 15, 2474-2489. |
| [37] |
Santamaría J, Bayman P (2005) Fungal epiphytes and endophytes of coffee leaves (Coffea arabica). Microbial Ecology, 50, 1-8.
PMID |
| [38] | Sha XL, Liang SX, Zhuang XL, Han QL, Bai ZH (2017) Nitrogen-fixing bacteria in the phyllosphere. Microbiology China, 44, 2443-2451. (in Chinese with English abstract) |
| [ 沙小玲, 梁胜贤, 庄绪亮, 韩庆莉, 白志辉 (2017) 植物叶际固氮菌研究进展. 微生物学通报, 44, 2443-2451.] | |
| [39] |
Singh P, Santoni S, Weber A, This P, Péros JP (2019) Understanding the phyllosphere microbiome assemblage in grape species (Vitaceae) with amplicon sequence data structures. Scientific Reports, 9, 14294.
DOI PMID |
| [40] |
Sloan WT, Lunn M, Woodcock S, Head IM, Nee S, Curtis TP (2006) Quantifying the roles of immigration and chance in shaping prokaryote community structure. Environmental Microbiology, 8, 732-740.
PMID |
| [41] | Song L, Fan J, Harris W, Wu S, Zhong H, Zhou Y, Wang N, Zhu X (2012) Adaptive characteristics of grassland community structure and leaf traits along an altitudinal gradient on a subtropical mountain in Chongqing, China. Plant Ecology, 213, 89-101. |
| [42] | Sun X, Guo LD, Hyde KD (2011) Community composition of endophytic fungi in Acer truncatum and their role in decomposition. Fungal Diversity, 47, 85-95. |
| [43] | Trivedi P, Leach JE, Tringe SG, Sa TM, Singh BK (2020) Plant-microbiome interactions: From community assembly to plant health. Nature Reviews Microbiology, 18, 607-621. |
| [44] |
Vincent JB, Weiblen GD, May G (2016) Host associations and beta diversity of fungal endophyte communities in New Guinea rainforest trees. Molecular Ecology, 25, 825-841.
DOI PMID |
| [45] |
Vorholt JA (2012) Microbial life in the phyllosphere. Nature Reviews Microbiology, 10, 828-840.
DOI PMID |
| [46] | Voříšková J, Baldrian P (2013) Fungal community on decomposing leaf litter undergoes rapid successional changes. The ISME Journal, 7, 477-486. |
| [47] | Wang LL, Liu MH, Li QH, Xu J (2016) Architecture characteristics of Helianthemum songaricum under different habitats. Journal of Desert Research, 36, 651-658. (in Chinese with English abstract) |
|
[ 王林龙, 刘明虎, 李清河, 徐军 (2016) 不同生境半日花(Helianthemum songaricum)植物构型特征. 中国沙漠, 36, 651-658.]
DOI |
|
| [48] |
Wang X, Yang T, Mao ZK, Lin F, Ye J, Fang S, Dai GH, Hu JR, Hao ZQ, Wang XG, Yuan ZQ (2022) Community structure of phyllosphere fungi associated with dominant tree species in a broad-leaved Korean pine forest of Changbai Mountain, Northeast China. Chinese Journal of Applied Ecology, 33, 2405-2412. (in Chinese with English abstract)
DOI |
|
[ 王星, 杨腾, 毛子昆, 蔺菲, 叶吉, 房帅, 戴冠华, 胡家瑞, 郝占庆, 王绪高, 原作强 (2022) 长白山阔叶红松林优势树种叶际真菌群落结构. 应用生态学报, 33, 2405-2412.]
DOI |
|
| [49] |
Wei Y, Lan G, Wu Z, Chen B, Quan F, Li M, Sun S, Du H (2022) Phyllosphere fungal communities of rubber trees exhibited biogeographical patterns, but not bacteria. Environmental Microbiology, 24, 3777-3790.
DOI PMID |
| [50] | Wu ZY (1980) Vegetation of China. Science Press, Beijing. (in Chinese) |
| [ 吴征镒 (1980) 中国植被. 科学出版社, 北京.] | |
| [51] |
Xie J, Wang XQ, Xu JW, Xie HW, Cai YH, Liu YZ, Ding X (2021) Strategies and structure feature of the aboveground and belowground microbial community respond to drought in wild rice (Oryza longistaminata). Rice, 14, 79.
DOI PMID |
| [52] |
Xu NH, Zhao QQ, Zhang ZY, Zhang Q, Wang Y, Qin GY, Ke MJ, Qiu DY, Peijnenburg WJGM, Lu T, Qian HF (2022) Phyllosphere microorganisms: Sources, drivers, and their interactions with plant hosts. Journal of Agricultural and Food Chemistry, 70, 4860-4870.
DOI PMID |
| [53] | Yan K, Han W, Zhu Q, Li C, Dong Z, Wang Y (2022) Leaf surface microtopography shaping the bacterial community in the phyllosphere: Evidence from 11 tree species. Microbiological Research, 254, 126897. |
| [54] | Yan X, Wang X, Liu XL, Li XW (2020) Study on the floristic genera and structure of the community of Helianthemum songaricum in Qingtongxia City, Ningxia. Journal of Agricultural Sciences, 41(1), 92-96. (in Chinese with English abstract) |
| [ 闫秀, 王旭, 刘小龙, 李小伟 (2020) 宁夏青铜峡半日花群落区系组成与群落结构特征. 农业科学研究, 41(1), 92-96.] | |
| [55] | Yang T, Tedersoo L, Soltis PS, Soltis DE, Sun M, Ma Y, Ni Y, Liu X, Fu X, Shi Y, Lin H-Y, Zhao YP, Fu C, Dai CC, Gilbert JA, Chu H (2023) Plant and fungal species interactions differ between aboveground and belowground habitats in mountain forests of eastern China. Science China: Life Sciences, 66, 1134-1150. |
| [56] |
Yao H, Sun X, He C, Maitra P, Li XC, Guo LD (2019) Phyllosphere epiphytic and endophytic fungal community and network structures differ in a tropical mangrove ecosystem. Microbiome, 7, 57.
DOI PMID |
| [57] | Yin Y, Wang YF, Cui HL, Zhou R, Li L, Duan GL, Zhu YG (2023) Distinctive structure and assembly of phyllosphere microbial communities between wild and cultivated rice. Microbiology Spectrum, 11, e0437122. |
| [58] | Zambell CB, White JF (2015) In the forest vine Smilax rotundifolia, fungal epiphytes show site-wide spatial correlation, while endophytes show evidence of niche partitioning. Fungal Diversity, 75, 279-297. |
| [59] | Zhou H, Gao Y, Jia XH, Wang MM, Ding JJ, Cheng L, Bao F, Wu B (2020) Network analysis reveals the strengthening of microbial interaction in biological soil crust development in the Mu Us Sandy Land, northwestern China. Soil Biology and Biochemistry, 144, 107782. |
| [60] | Zhu C, Lin Y, Wang Z, Luo W, Zhang Y, Chu C (2023a) Community assembly and network structure of epiphytic and endophytic phyllosphere fungi in a subtropical mangrove ecosystem. Frontiers in Microbiology, 14, 1147285. |
| [61] | Zhu J, Sun X, Tang QY, Zhang ZD (2021) Seasonal dynamics and persistency of endophyte communities in Kalidium schrenkianum shifts under radiation stress. Frontiers in Microbiology, 12, 778327. |
| [62] | Zhu K, Zhu W, Zhang W, Liu J, Ding C (2023b) Characteristics of phyllosphere microbial communities associated with three different plants in the semi-arid areas of Northwest Liaoning. Journal of Soil Science and Plant Nutrition, 23, 2066-2079. |
| [63] |
Zhu L, Hu Y, Zhao X, Zhao P, Ouyang L, Ni G, Liu N (2019) Specific responses of sap flux and leaf functional traits to simulated canopy and understory nitrogen additions in a deciduous broadleaf forest. Functional Plant Biology, 46, 986-993.
DOI PMID |
| No related articles found! |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
备案号:京ICP备16067583号-7
Copyright © 2022 版权所有 《生物多样性》编辑部
地址: 北京香山南辛村20号, 邮编:100093
电话: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn