



Biodiv Sci ›› 2018, Vol. 26 ›› Issue (5): 519-526. DOI: 10.17520/biods.2018038 cstr: 32101.14.biods.2018038
• Original Papers • Previous Articles Next Articles
Juan Wang, Yaxin Zhai, Aiqin Zhang*( )
)
Received:2018-02-05
Accepted:2018-04-26
Online:2018-05-20
Published:2018-09-11
Contact:
Zhang Aiqin
About author:# Co-first authors
Juan Wang, Yaxin Zhai, Aiqin Zhang. Temporal variation of plant sexes in a wild population of Tulipa sinkiangensis over seven years[J]. Biodiv Sci, 2018, 26(5): 519-526.
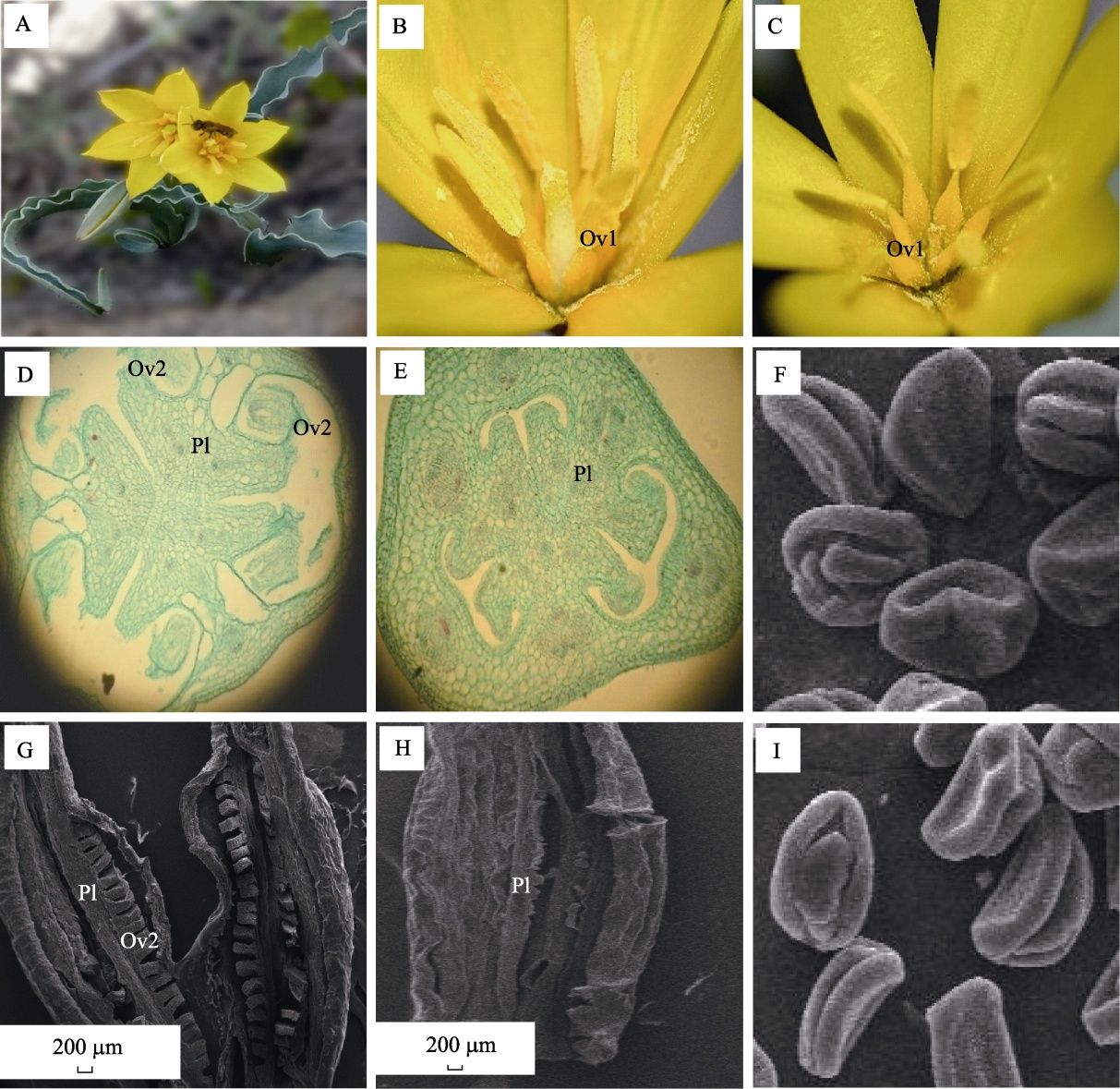
Fig. 1 The morphological and anatomy structure of staminate flower and perfect flower in Tulipa sinkiangensis. (A) Tulipa sinkiangensis plant with perfect flower; (B) Perfect flower with fertile ovary; (C) Staminate flower with sterile ovary; (D) Ovary crosscutting of perfect flower; (E) Ovary crosscutting of staminate flower; (F) Pollen grain morphology of perfect flower; (G) Ovary longitudinal cutting of perfect flower; (H) Ovary longitudinal cutting of staminate flower; (I) Pollen grain morphology of staminate flower. Ov1, Ovary; Ov2, Ovule; Pl, Placenta.
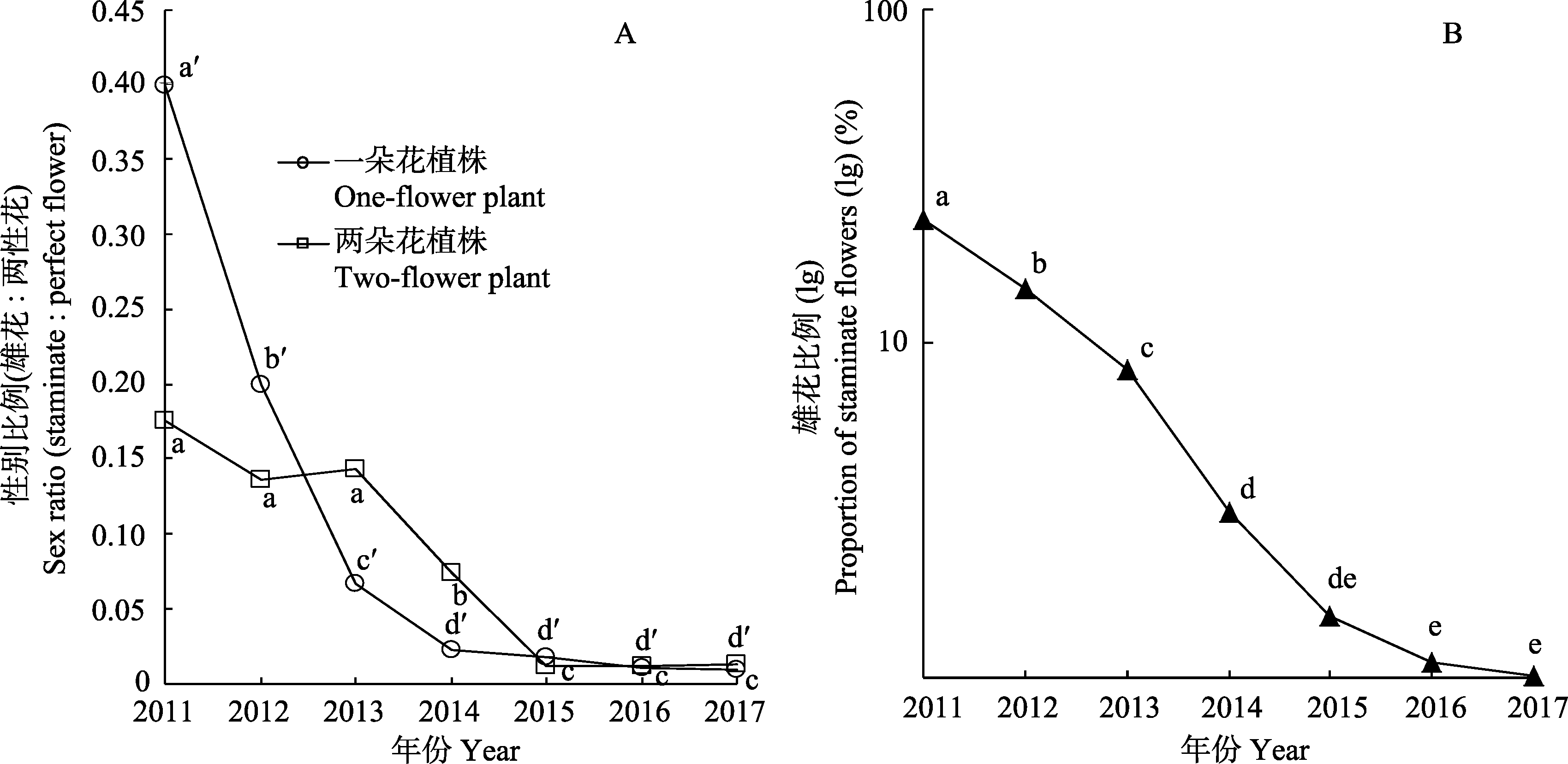
Fig. 2 Temporal variation of plant sexes in a wild population of Tulipa sinkiangensis. (A) The sex-ratio dynamic of staminate and perfect flower within plants from 2011 to 2017; (B) Annual changes in the proportion of staminate flowers at the level of population from 2011 to 2017. Different letters indicate significant difference at P = 0.05 level.
| 花型 Floral morph | 外轮Outer (cm) | 内轮Inner (cm) | 雄蕊Stamen (cm) | 雌蕊Pistil (cm) | 花粉量 No. of pollen grains per flower | 败育率 Abortin ratio (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 花瓣长 Petal length | 花瓣宽 Petal width | 花瓣长 Petal length | 花瓣宽 Petal width | 花丝长 Filament length | 花药长 Anther length | 子房直径 Ovary diameter | 雌蕊长 Pistil length | |||
| 两性花 Perfect flower | 2.31 ± 0.050 | 0.81 ± 0.035 | 2.14 ± 0.042 | 0.97 ± 0.042 | 0.52 ± 0.009 | 0.57 ± 0.013 | 0.22 ± 0.011 | 0.82 ± 0.039 | 101,000 ± 7,007 | 3.16 ± 0.99 |
| 雄花 Staminate flower | 2.20 ± 0.039 | 0.59 ± 0.023 | 1.99 ± 0.040 | 0.84 ± 0.034 | 0.50 ± 0.010 | 0.51 ± 0.015 | 0.08 ± 0.010 | 0.56 ± 0.045 | 95,055 ± 10,249 | 3.47 ± 0.91 |
| t | 1.79 | 5.38 | 2.64 | 2.38 | 1.35 | 3.52 | 8.75 | 4.39 | 0.48 | 0.22 |
| P | 0.080 | 2.15*10-6 | 0.011 | 0.021 | 0.18 | 0.0010 | 4.77*10-7 | 0.0010 | 0.64 | 0.83 |
Table 1 Floral traits of perfect and staminate flowers in Tulipa sinkiangensis (mean ± SE, n = 20)
| 花型 Floral morph | 外轮Outer (cm) | 内轮Inner (cm) | 雄蕊Stamen (cm) | 雌蕊Pistil (cm) | 花粉量 No. of pollen grains per flower | 败育率 Abortin ratio (%) | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| 花瓣长 Petal length | 花瓣宽 Petal width | 花瓣长 Petal length | 花瓣宽 Petal width | 花丝长 Filament length | 花药长 Anther length | 子房直径 Ovary diameter | 雌蕊长 Pistil length | |||
| 两性花 Perfect flower | 2.31 ± 0.050 | 0.81 ± 0.035 | 2.14 ± 0.042 | 0.97 ± 0.042 | 0.52 ± 0.009 | 0.57 ± 0.013 | 0.22 ± 0.011 | 0.82 ± 0.039 | 101,000 ± 7,007 | 3.16 ± 0.99 |
| 雄花 Staminate flower | 2.20 ± 0.039 | 0.59 ± 0.023 | 1.99 ± 0.040 | 0.84 ± 0.034 | 0.50 ± 0.010 | 0.51 ± 0.015 | 0.08 ± 0.010 | 0.56 ± 0.045 | 95,055 ± 10,249 | 3.47 ± 0.91 |
| t | 1.79 | 5.38 | 2.64 | 2.38 | 1.35 | 3.52 | 8.75 | 4.39 | 0.48 | 0.22 |
| P | 0.080 | 2.15*10-6 | 0.011 | 0.021 | 0.18 | 0.0010 | 4.77*10-7 | 0.0010 | 0.64 | 0.83 |
| 1 | Anderson GJ, Symon DE (1989) Functional dioecy and andromonoecy in Solanum. Evolution, 43, 204-219. |
| 2 | Abdusalam A, Tan DY, Tahan O (2012) Effects of vegetative growth, plant size and flowering order on sexual reproduction allocation of Tulipa sinkiangensis. Biodiversity Science, 20, 391-399. (in Chinese with English abstract) |
| [艾沙江·阿不都沙拉木, 谭敦炎, 吾买尔夏提·塔汉 (2012) 新疆郁金香营养生长、个体大小和开花次序对繁殖分配的影响. 生物多样性, 20, 391-399.] | |
| 3 | Abdusalam A, Tan DY (2014) Contribution of temporal floral closure to reproductive success of the spring-flowering Tulipa iliensis. Journal of Systematics and Evolution, 52, 186-194. |
| 4 | Bertin RI (1982) The evolution and maintenance of andromonoecy. Evolutionary Theory, 6, 25-32. |
| 5 | Charnov EL (1982) The Theory of Sex Allocation. Princeton University Press, Princeton. |
| 6 | Charlesworth D (1999) Theories of the evolution of dioecy. In: Gender and Sexual Dimorphism in Flowering Plants (eds Geber MA, Dawson TE, Delph LF), pp. 33-60. Springer, Berlin. |
| 7 | Chen JR (1991) The review and application of plant sporopollen dyeing technology. In: Botanical Research (ed. Institute of Botany, Chinese Academy of Sciences), pp. 269-276. Science Press, Beijing. (in Chinese) |
| [陈家瑞 (1991) 植物孢粉染色技术综述及其应用. 植物学集刊(中国科学院植物研究所编). 科学出版社, 北京.] | |
| 8 | Dai C, Galloway LF (2012) Male flowers are better fathers than hermaphroditic flowers in andromonoecious Passiflora incarnate. New Phytologist, 193, 787-796. |
| 9 | Darwin C (1877) The Different Forms of Flowers on Plants of the Same Species. John Murray, London. |
| 10 | Delesalle VA (1989) Year-to-year changes in phenotypic gender: A monoecious cucurbit Apodanthera undulata. American Journal of Botany, 76, 30-39. |
| 11 | Diggle PK (1993) Developmental plasticity, genetic variation, and the evolution of andromonoecy in Solanum hirtum (Solanaceae). American Journal of Botany, 80, 967-973. |
| 12 | Elle E (1999) Sex allocation and reproductive success in the andromonoecious perennial Solanum carolinense (Solanaceae). I. Female success. American Journal of Botany, 86, 278-286. |
| 13 | Emms SK (1993) Andromonoecy in Zigadenus paniculatus (Liliaceae): Spatial and temporal patterns of sex allocation. American Journal of Botany, 80, 914-923. |
| 14 | Freeman DC, Harper KT, Charnov EL (1980) Sex change in plants: Old and new observations and new hypotheses. Oecologia, 47, 222-232. |
| 15 | Han B, Wang XF, Huang SQ (2011) The production of male flowers does not decrease with plant size in insect-pollinated Sagittaria trifolia, contrary to predictions of size-dependent sex allocation. Journal of Systematics and Evolution, 49, 379-385. |
| 16 | Hanzawa FM, Kalisz S (1993) The relationship between age, size, and reproduction in Trillum grandiflorum (Liliaceae). American Journal of Botany, 80, 405-410. |
| 17 | Heslop-Harrison J (1958) The unisexual flower: A reply to criticism. Phytomorphology, 8, 117-184. |
| 18 | Huang SQ (2003) Flower dimorphism and the maintenance of andromonoecy in Sagittaria guyanensis ssp. lappula (Alismataceae). New Phytologist, 157, 357-364. |
| 19 | Huang SQ, Guo YH (2000) Advances in the research of pollination biology. Chinese Science Bulletin, 45, 225-237. (in Chinese) |
| [黄双全, 郭友好 (2000) 传粉生物学的研究进展. 科学通报, 45, 225-237.] | |
| 20 | Huang SQ, Sun SG, Takahashi Y, Guo YH (2002) Gender variation of sequential inflorescences in a monoecious plant Sagittaria trifolia (Alismataceae). Annals of Botany, 90, 613-622. |
| 21 | Irwin RE (2000) Morphological variation and female reproductive success in tow sympatric Trillium species: Evidence for phenotypic selection in Trillium erectum and Trillium grandiflorum (Liliaceae). American Journal of Botany, 87, 205-214. |
| 22 | Kamenetsky R (1994) Life cycle, flower initiation, and propagation of the desert geophytes Allium rothii. International Journal of Plant Sciences, 49, 603-609. |
| 23 | Kawano S, Hiratsuka A, Hayashi K (1982) Life history characteristics and survivorship of Erythronium japonicum. Oikos, 38, 129-149. |
| 24 | Kawano S, Masuda J, Hayashi K (2008) Life-history monographs of Japanese plants. 10: Friitillaria koidzumiana Ohwi (Liliaceae). Plant Species Biology, 23, 51-57. |
| 25 | Liao WJ, Zhang QG, Zhang DY (2003) A preliminary study on the reproductive features of Nigrum along an altitudinal gradient. Acta Phytoecologica Sinica, 27, 240-248. (in Chinese with English abstract) |
| [廖万金, 张全国, 张大勇 (2003)不同海拔藜芦种群繁殖特征的初步研究. 植物生态学报, 27, 240-248.] | |
| 26 | Lloyd DG (1982) Selection of combined versus separate sexes in seed plants. The American Naturalist, 120, 571-585. |
| 27 | Lloyd DG, Bawa KS (1984) Modification of the gender of seed plants in varying conditions. Evolutionary Biology, 17, 255-338. |
| 28 | Mamut J, Xiong YZ, Tan DY, Huang SQ (2014) Pistillate flowers experience more pollen limitation and less geitonogamy than perfect flowers in a gynomonoecious herb. New Phytologist, 201, 670-677. |
| 29 | Mamut J, Xiong YZ, Tan DY, Huang SQ (2017) Flexibility of resource allocation in a hermaphrodite-gynomonoecious herb through deployment of female and male resources in perfect flowers. American Journal of Botany, 104, 461-467. |
| 30 | Mao ZM (1984) The research of Tulipa L. plants. Arid Zone Research, 1(2), 39-43. (in Chinese) |
| [毛祖美 (1984) 新疆郁金香属植物的研究. 干旱区研究, 1(2), 39-43.] | |
| 31 | Pannell JR, Ojeda F (2000) Patterns of flowering and sex-ratio variation in the Mediterranean shrub Phillyrea angustifolia (Oleaceae): Implications for the maintenance of males with perfects. Ecology Letters, 3, 495-502. |
| 32 | Primack RB, Lloyd DG (1980) Andromonoecy in the New- Zealand montane shrub manuka, Leptospermum scoparium (Myrtaceae). American Journal of Botany, 67, 361-368. |
| 33 | Shimizu T, Hatanaka Y, Zentoh H, Yashima T, Kinoshita E, Watano Y, Shimizu T (1998) The role of sexual and clonal reproduction in maintaining population in Fritillaria camtschatcensis (L.) Ker-Gawl. (Liliaceae). Ecological Research, 13, 27-39. |
| 34 | Sinclair JP, Kameyama Y, Shibata A, Kudo G (2016) Male- biased hermaphrodites in a gynodioecious shrub, Daphne jezoensis. Plant Biology, 18, 859-867. |
| 35 | Stócklin J, Pavre P (1994) Effects of plant size and morphological constraints on variation in reproductive components in two related species of Epilobium. Journal of Ecology, 82, 735-746. |
| 36 | Traveset A (1995) Reproductive ecology of Cneorum tricoccon L. (Cneoraceae) in the Balearic-Islands. Botanical Journal of the Linnean Society, 117, 221-232. |
| 37 | Weiner J (1988) The influence of competition on plant reproduction. In: Plant Reproductive Ecology: Patterns and Strategies (eds Lovett DJ, Lovett DL), pp. 228-245. Oxford University Press, New York. |
| 38 | Zhang DY (2004) Life History Evolution and Reproductive Ecology in Plants. Science Press, Beijing. (in Chinese) |
| [张大勇 (2004) 植物生活史进化与繁殖生态学. 科学出版社, 北京.] | |
| 39 | Zhang ZC, Tan DY (2012) Floral sex allocation and flowering pattern in the andromonocious Soranthus meyeri (Apiaceae). Chinese Journal of Plant Ecology, 36, 63-71. (in Chinese with English abstract) |
| [张振春, 谭敦炎 (2012) 雄全同株植物簇花芹花期性别分配与开花式样. 植物生态学报, 36, 63-71.] | |
| 40 | Zhang ZQ, Zhu XF, Sun H, Yang YP, Barrett SCH (2014) Size-dependent gender modification in Lilium apertum (Liliaceae): Does this species exhibit gender diphasy? Annals of Botany, 114, 441-453. |
| [1] | Weijie Shu, Hua He, Luo Zeng, Zhirong Gu, Dunyan Tan, Xiaochen Yang. Spatial distribution and sexual dimorphism of dioecious Arisaema erubescens [J]. Biodiv Sci, 2024, 32(6): 24084-. |
| [2] | Yaobin Song, Li Xu, Junpeng Duan, Weijun Zhang, Xiaolu Shentu, Tianxiang Li, Runguo Zang, Ming Dong. Sex ratio and spatial pattern of Taxus fuana, a Wild Plant with Extremely Small Populations in Tibet [J]. Biodiv Sci, 2020, 28(3): 269-276. |
| [3] | Aysajan Abdusalam, Dunyan Tan, Omarxat Tahan. Effects of vegetative growth, plant size and flowering order on sexual reproduction allocation of Tulipa sinkiangensis [J]. Biodiv Sci, 2012, 20(3): 391-399. |
| [4] | Lizhe Cai, Xinwei Chen, Chen Wu, Xin Peng, Jing Cao, Sujing Fu. Temporal and spatial variation of macrofaunal communities in Shenzhen Bay intertidal zone between 1995 and 2010 [J]. Biodiv Sci, 2011, 19(6): 702-709. |
| [5] | Chunfang Pan, Chunyu Zhang, Xiuhai Zhao, Fucai Xia, Haicheng Zhou, Yun Wang. Sex ratio and spatial patterns of males and females of different ages in the dioecious understory tree, Acer barbinerve, in a broad-leaved Korean pine forest [J]. Biodiv Sci, 2010, 18(3): 292-299. |
| [6] | GU Hai-Yan, YANG Da-Rong. Community structure and species diversity of fig wasps from Ficus altissima [J]. Biodiv Sci, 2003, 11(3): 188-196. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn ![]()