



Biodiv Sci ›› 2016, Vol. 24 ›› Issue (1): 95-101. DOI: 10.17520/biods.2015195 cstr: 32101.14.biods.2015195
Special Issue: 传粉生物学
• Reviews • Previous Articles Next Articles
Zhenna Qian1,2, Mingxun Ren1,2,*( )
)
Received:2015-07-06
Accepted:2015-09-15
Online:2016-01-20
Published:2016-06-12
Contact:
Ren Mingxun
Zhenna Qian, Mingxun Ren. Floral evolution and pollination shifts of the “Malpighiaceae route” taxa, a classical model for biogeographical study[J]. Biodiv Sci, 2016, 24(1): 95-101.
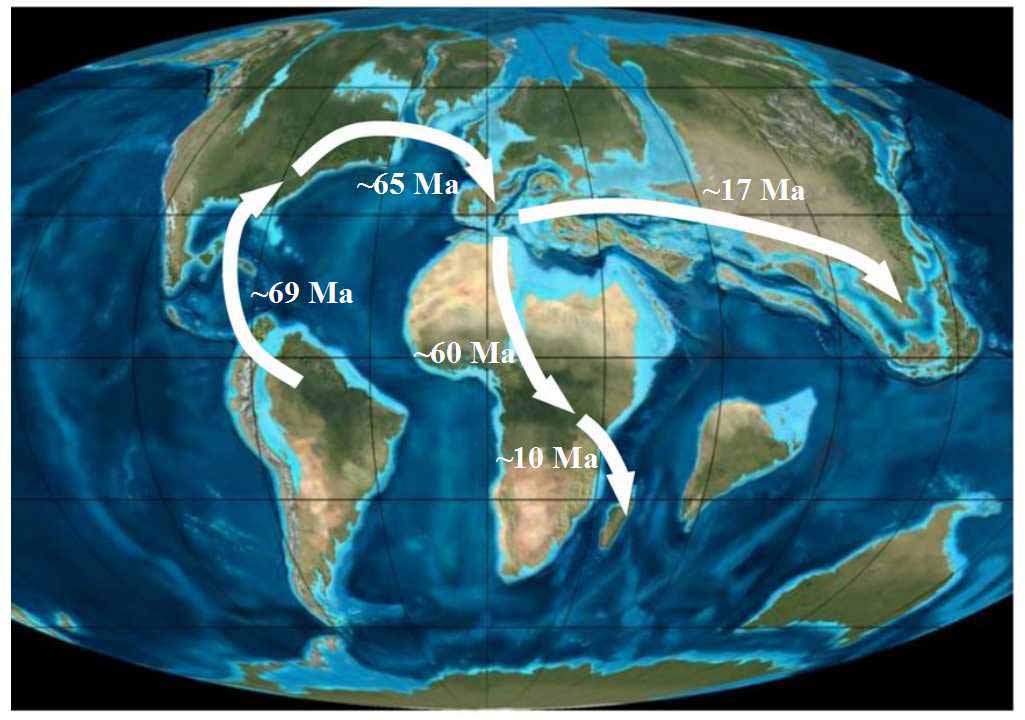
Fig. 1 The sketch map of “Malpighiaceae route”. The family is proposed to be originated at about 75 Ma at the north of South America and its current distributions are proposed to have resulted by migration from South America to the Old World via land bridges in North Atlantic Ocean during Eocene (~ 65 Ma). The time near the dispersal route is adapted from Davis et al (2010, 2014).
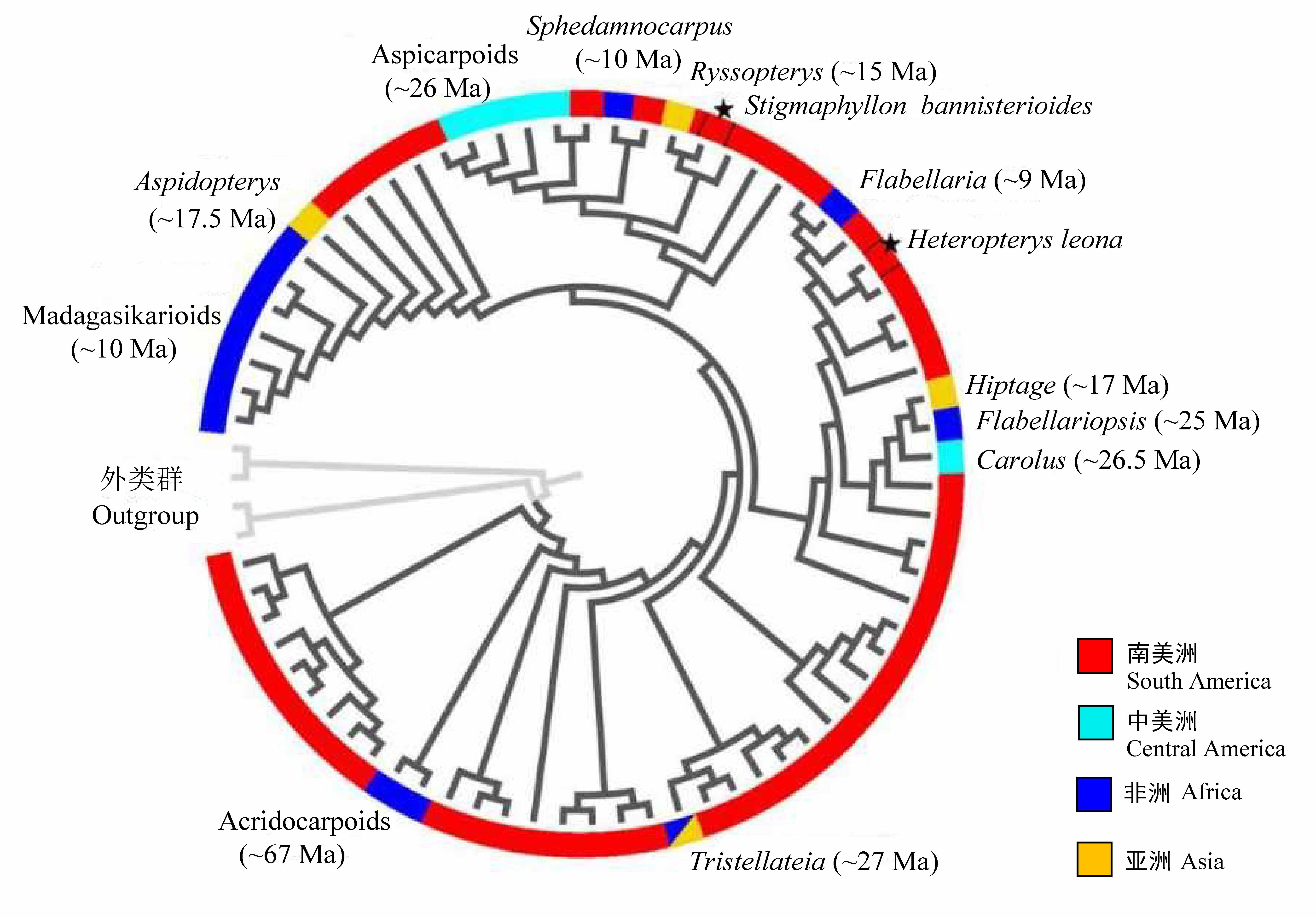
Fig. 2 Malpighiaceae migrate independently into Old World (Africa and Asia) from South and Central America a total of seven times in the ancient time, with two very recent arrives to the west coast of Africa directly across the Atlantic Ocean (★). Phylogeny tree and the dispersal time are obtained from Davis et al (2014).
| 繁育系统 Breeding system | 花对称性 Floral symmetry | 花萼腺体 Calyx glands | 传粉者 Pollinators | 参考文献 References | |
|---|---|---|---|---|---|
| 南美洲 South America | 两性花 Hermaphrodite | 两侧对称 Bilateral symmetry | 10个油脂腺体 (每个萼片2个) 10 oil glands, paired on each sepal | 美洲特有的条蜂科集油蜂 America-endemic oil-collecting bees, including Centridini, Tetrapediini, and Tapinotaspidini | Anderson, 1979; Davis & Anderson, 2010; Davis et al, 2014 |
| 中美洲、北美洲 Central and North America | 两性花(闭花受精) Hermaphrodite (cleistogamy) | 辐射对称 Radial symmetry | 无 None | 花内自交, 不需传粉者 Autogamy (pollinator not needed) | Anderson, 1980 |
| 非洲 Africa | 雄花两性花异株(功能性雌雄异株) Androdioecy (functional dioecy) | 辐射对称 Radial symmetry | 无 None | 收集花粉的蜜蜂科昆虫 Pollen-gathering bees | Davis, 2002; Davis et al, 2014 |
| 亚洲 Asia | 两性花(镜像花) Hermaphrodite (mirror- image flowers) | 两侧对称 Bilateral symmetry | 无或1 (如有, 分泌糖) None or single (if present, secretes nectar) | 收集花粉的大蜜蜂(Apis dorsata) Asian giant bees (Apis dorsata) collecting pollen | Ren et al, 2013; Ren, 2015 |
Table 1 Breeding system, floral syndromes and pollinators of Malpighiaceae in different continents
| 繁育系统 Breeding system | 花对称性 Floral symmetry | 花萼腺体 Calyx glands | 传粉者 Pollinators | 参考文献 References | |
|---|---|---|---|---|---|
| 南美洲 South America | 两性花 Hermaphrodite | 两侧对称 Bilateral symmetry | 10个油脂腺体 (每个萼片2个) 10 oil glands, paired on each sepal | 美洲特有的条蜂科集油蜂 America-endemic oil-collecting bees, including Centridini, Tetrapediini, and Tapinotaspidini | Anderson, 1979; Davis & Anderson, 2010; Davis et al, 2014 |
| 中美洲、北美洲 Central and North America | 两性花(闭花受精) Hermaphrodite (cleistogamy) | 辐射对称 Radial symmetry | 无 None | 花内自交, 不需传粉者 Autogamy (pollinator not needed) | Anderson, 1980 |
| 非洲 Africa | 雄花两性花异株(功能性雌雄异株) Androdioecy (functional dioecy) | 辐射对称 Radial symmetry | 无 None | 收集花粉的蜜蜂科昆虫 Pollen-gathering bees | Davis, 2002; Davis et al, 2014 |
| 亚洲 Asia | 两性花(镜像花) Hermaphrodite (mirror- image flowers) | 两侧对称 Bilateral symmetry | 无或1 (如有, 分泌糖) None or single (if present, secretes nectar) | 收集花粉的大蜜蜂(Apis dorsata) Asian giant bees (Apis dorsata) collecting pollen | Ren et al, 2013; Ren, 2015 |
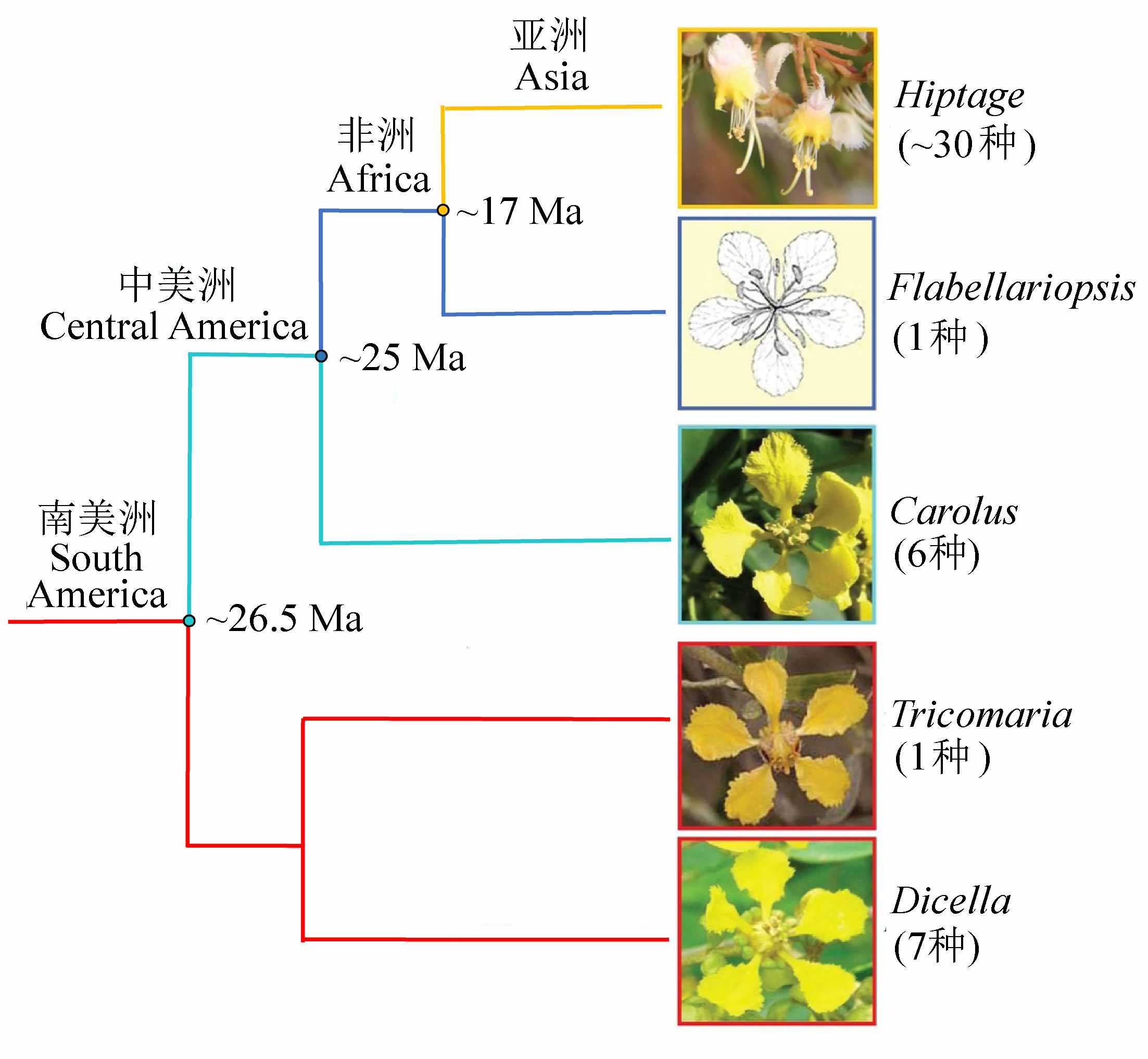
Fig. 3 The Asia-endemic Hiptage is in the clade with complete migration history of Malpighiaceae route, having endemic genera respectively in America, Africa, and Asia. The number in the brackets is the species diversity of the genus. Phylogeny relationships are determined according to Davis & Anderson (2010).
| 1 | Anderson WR (1979) Floral conservatism in neotropical Malpighiaceae. Biotropica, 11, 219-223. |
| 2 | Anderson WR (1980) Cryptic self-fertilization in the Malpighiaceae. Science, 207, 892-893. |
| 3 | Anderson WR (1981) Malpighiaceae in the botany of the Guayana Highland. Part XI. Memoirs of the New York Botanical Garden, 32, 45-48. |
| 4 | Anderson WR (1990) The origin of the Malpighiaceae: the evidence from morphology. Memoirs of the New York Botanical Garden, 64, 210-224. |
| 5 | Anderson WR, Anderson C, Davis CC (2006) Malpighiaceae.[accessed <date-in-citation content-type="access-date">2015-06-19</date-in-citation>] |
| 6 | Cappellari SC, Haleem MA, Marsaioli AJ, Tidon R, Simpson BB (2011) Pterandra pyroidea: a case of pollination shift within Neotropical Malpighiaceae. Annals of Botany, 107, 1323-1334. |
| 7 | Chanderbali AS, van der Werff H, Renner SS (2001) Phylogeny and historical biogeography of Lauraeeae: evidence from the chloroplast and nuclear genomes. Annals of Missouri Botanical Garden, 88, 104-134. |
| 8 | Chen SK, Funton AM (2008) Malpighiaceae. In: Flora of China, Volume 11 (Eds Wu ZY, Raven PH, Hong DY), pp. 135-138. Science Press, Beijing & Missouri Botanical Garden Press, St. Luis. |
| 9 | Davis CC (2002) Madagasikaria (Malpighiaceae): a new genus from Madagascar with implications for floral evolution in Malpighiaceae. American Journal of Botany, 89, 699-706. |
| 10 | Davis CC, Bell CD, Mathews S, Donoghue MJ (2002) Laurasian migration explains Gondwanan disjunctions: evidence from Malpighiaceae. Proceedings of the National Academy of Sciences, USA, 99, 6833-6837. |
| 11 | Davis CC, Anderson WR (2010) A complete generic phylogeny of Malpighiaceae inferred from nucleotide sequence data and morphology. American Journal of Botany, 97, 2031-2048. |
| 12 | Davis CC, Schaefer H, Xi ZX, Baum DA, Donoghue MJ, Harmon LJ (2014) Long-term morphological stasis maintained by a plant-pollinator mutualism. Proceedings of the National Academy of Sciences, USA, 111, 5914-5919. |
| 13 | Fritseh PW (2001) Phylogeny and biogeography of the flowering plant genus Styrax (Styraeaceae) based on chloroplast DNA restriction sites and DNA sequences of the internal transeribed spacer region. Molecular Phylogenetics and Evolution, 19, 387-408. |
| 14 | Gates B (1982) Banisteriopsis, Diplopterys (Malpighiaceae). Flora Neotropica, 30, 1-237. |
| 15 | Jesson LK, Barrett SCH (2002) Solving the puzzle of mirror-image flowers. Nature, 417, 707. |
| 16 | Jesson LK, Barrett SCH (2003) The comparative biology of mirror-image flowers. International Journal of Plant Sciences, 164, 237-249. |
| 17 | Lin Y, Tan DY (2007) Enantiostyly in angiosperms and its evolutionary significance. Acta phytotaxonomica Sinica, 45, 901-916. (in Chinese with English abstract) |
| [林玉, 谭敦炎(2007) 被子植物镜像花柱及其进化意义. 植物分类学报, 45, 901-916.] | |
| 18 | Ren MX (2015) The upper reaches of the largest river in Southern China as an ‘evolutionary front’ of tropical plants: evidences from Asia-endemic genus Hiptage (Malpighiaceae). Collectanea Botanica, 34, e002. |
| 19 | Ren MX, Zhang DY (2004) Herkogamy. In: Plant Life-History Evolution and Reproductive Ecology (ed. Zhang DY), pp. 303-320. Science Press, Beijing. (in Chinese) |
| [任明迅, 张大勇 (2004) 雌雄异位. 见: 植物生活史进化与繁殖生态学 (张大勇主编). 科学出版社, 北京.] | |
| 20 | Ren MX, Zhong YF, Song XQ (2013) Mirror-image flowers without buzz pollination in the Asia-endemic Hiptage benghalensis (Malpighiaceae). Botanical Journal of the Linnean Society, 173, 764-774. |
| 21 | Renner SS, Clausing G, Meyer K (2001) Historical biogeography of Melastomataceae: the roles of tertiary migration and long-distance dispersal. American Journal of Botany, 88, 1290-1300. |
| 22 | Renner SS, Schaefer H (2010) The evolution and loss of oil-offering flowers: new insights from dated phylogenies for angiosperms and bees. Philosophical Transactions of the Royal Society of London, Series B, 365, 423-435. |
| 23 | Steiner KE (1985) Functional dioecism in the Malpighiaceae: The breeding system of Spachea membranacea Cuatr. American Journal of Botany, 72, 1537-1543. |
| 24 | Vogel S (1990) History of the Malpighiaceae in the light of pollination ecology. Memoirs of the New York Botanical Garden, 55, 130-142. |
| 25 | Week A, Daly AW, Simpson BB (2005) The phylogenetic history and biogeography of the frankincense and myrrh family (Burseraeeae) based on nuclear and chloroplast sequence data. Molecular Phylogenetics and Evolution, 35, 85-101. |
| 26 | Zhang WH, Kramer EM, Davis CC (2010) Floral symmetry genes and the origin and maintenance of zygomorphy in a plant-pollinator mutualism. Proceedings of the National Academy of Sciences, USA, 107, 6388-6393. |
| 27 | Zhou ZK, Yang XF, Yang QS (2006) Land bridge and long-distance dispersal—old ideas, new evidence. Chinese Science Bulletin, 51, 897-886. (in Chinese) |
| [周浙昆, 杨雪飞, 杨青松 (2006) 陆桥说和长距离扩散——老观点, 新证据. 科学通报, 51, 879-886.] |
| [1] | Zhaoyang Jing, Keguang Cheng, Heng Shu, Yongpeng Ma, Pingli Liu. Whole genome resequencing approach for conservation biology of endangered plants [J]. Biodiv Sci, 2023, 31(5): 22679-. |
| [2] | Ting Wang, Zengqiang Xia, Jiangping Shu, Jiao Zhang, Meina Wang, Jianbing Chen, Kanglin Wang, Jianying Xiang, Yuehong Yan. Dating whole-genome duplication reveals the evolutionary retardation of Angiopteris [J]. Biodiv Sci, 2021, 29(6): 722-734. |
| [3] | Simiao Sun, Jixin Chen, Weiwei Feng, Chang Zhang, Kai Huang, Ming Guan, Jiankun Sun, Mingchao Liu, Yulong Feng. Plant strategies for nitrogen acquisition and their effects on exotic plant invasions [J]. Biodiv Sci, 2021, 29(1): 72-80. |
| [4] | Zhenna Qian, Qianwan Meng, Mingxun Ren. Pollination ecotypes and herkogamy variation of Hiptage benghalensis (Malpighiaceae) with mirror-image flowers [J]. Biodiv Sci, 2016, 24(12): 1364-1372. |
| [5] | Qianghua Xu,Zhichao Wu,Liangbiao Chen. Biodiversity and adaptive evolution of Antarctic notothenioid fishes [J]. Biodiv Sci, 2014, 22(1): 80-87. |
| [6] | Bao-Rong Lu, Hui Xia, Wei Wang, Xiao Yang. Impacts of natural hybridization and introgression on biological invasion of plant species [J]. Biodiv Sci, 2010, 18(6): 577-589. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn ![]()