独栖蜂在分类学上属于膜翅目细腰亚目。它们最显著的生物学习性是独栖性, 即雌性独自产下后代, 建造巢穴, 并寻找食物资源来供养它们的后代(Danforth et al, 2019)。在自然条件下, 很多独栖蜂会选择在空腔洞穴中筑巢(Iwata, 1976), 场所可包括地面或石头上的孔洞、砖石缝隙、甲虫洞穴、蜗牛壳、空虫瘿, 以及形状与空腔洞穴接近的几乎所有其他场所(Rozen & Eickwort, 1997)。独栖蜂功能类群包括独栖蜜蜂(传粉者, 访花采粉)和捕食蜂(捕食者, 捕食鳞翅目幼虫、蜘蛛等各种节肢动物)。此外, 还有与独栖蜂密切相关的寄生类群。独栖蜂和寄生者是生态系统重要的组成部分, 与环境密切相关(Loyola & Martins, 2006)。
独栖蜂的分布更倾向于干燥的环境, 而非湿润的地区(Danforth et al, 2019)。温度是影响独栖蜂物种丰富度的重要因素, 通常温度越高物种丰富度越高(Mayr et al, 2020)。在草地生态系统中, 捕食蜂类群多样性与植物多样性直接正相关, 而独栖蜜蜂和寄生者多样性与植物多样性间接正相关(Ebeling et al, 2012)。在温带森林中, 尽管植物多样性对独栖蜜蜂和捕食蜂的物种丰富度没有影响, 但是它们的多度与植物多样性正相关, 同时它们天敌的丰富度和多度也增加(Sobek et al, 2009)。在亚热带森林系统中, 研究发现独栖蜜蜂的丰富度和多度与树种丰富度无关, 但是捕食蜂和寄生者的丰富度和多度与树种丰富度呈正相关(Guo et al, 2021); 独栖蜂与其寄生性天敌的多度以及树木的生物量和郁闭度正相关, 丰富度与树木多度正相关(Fornoff et al, 2021)。
独栖蜂是生态系统中重要的捕食者、传粉者, 有非常重要的生态功能和研究价值。巢管法为研究独栖蜂提供了良好的技术支持。在环境中设置巢管来模拟和替代独栖蜂在自然环境下的巢穴, 利用其在空腔洞穴中筑巢的习性, 吸引它们在巢管中筑巢, 以达到收集样本的目的(Tscharntke et al, 1998)。利用巢管的独栖蜂研究涉及生物分类学、生物生活史、生态学甚至农业生产等多个方面。自第一篇文章(Medler & Fye, 1956)发表到1980年前后, 巢管法主要用于研究独栖蜂生活史等生物学特性。1980年以后, 特别是1990年至今, 巢管法的相关研究大多以生态学为主(Staab et al, 2018)。
关于独栖蜂的生物学特性研究涉及温带、亚热带和热带几乎所有气候类型地区, 如南美洲热带和亚热带的森林、种植园、沼泽和草地等(Hermes et al, 2013; Buschini & Buss, 2014; da Costa & Buschini, 2016; Marinho et al, 2018); 北美洲亚热带和温带山区、沙地、森林、草地和果园等(Giles & Ascher, 2006; Cane et al, 2007); 欧洲亚热带和温带的森林、草地和农田(Moron et al, 2008; Polidori et al, 2010; Osorio et al, 2015; Tucker et al, 2019); 非洲热带森林、草原和乞力马扎罗山区(Longair, 2004; Mayr et al, 2020); 甚至包括大洋洲澳大利亚的亚热带地区(Paini, 2004)。研究类群包括蜜蜂科、切叶蜂科、胡蜂科的蜾蠃亚科、蛛蜂科、泥蜂科和方头泥蜂科, 而研究内容多是筑巢习性、季节动态、发生规律和寄生性天敌等生物学特性。对独栖蜂的研究遍布世界各地, 大部分研究集中在北半球的温带和南半球的热带地区, 主要有巴西(29.6%)、美国(24.7%)和德国(5.5%), 而对亚洲地区和亚热带地区的研究非常缺乏(Staab et al, 2018)。其中只有很少的研究在亚洲地区进行, 如越南亚热带地区的黃喙蜾蠃(Rhynchium brunneum)和倾秀蜾蠃(Pareumenes quadrispinosus) (Dang & Nguyen, 2019; Dang & Fateryga, 2021)、印度亚热带地区的Megachile studiosa (Kunjwal et al, 2021)、尼泊尔亚热带萨加玛塔国家公园的Ancistrocerus sikhimensis及其寄生性天敌(Boesi et al, 2005)等。在中国亚热带地区的研究更加稀少, 只有少数物种的生物学特性记录, 如捕食蜚蠊的Isodontia diodon (Barthélémy, 2010)、黄缘蜾蠃(Anterhynchium flavomarginatum) (窦飞越等, 2021)、日本佳盾蜾蠃(Euodynerus nipanicus) (黄敦元等, 2014)、缘叶舌蜂(Hylaeus perforata)及其天敌窄头褶翅蜂(Gasteruption corniculigerum) (郭鹏飞等, 2021)等。综上, 世界范围内对独栖蜂的研究多集中在单个物种的生物学特性方面, 仍缺乏对中国亚热带地区独栖蜂物种多样性和生物学特性的研究。
本研究基于世界上最大的亚热带树木多样性实验样地BEF-China (Bruelheide et al, 2014), 通过长期野外定点监测, 综合研究了中国亚热带森林地区独栖蜂及其寄生者的多样性和生物学特征, 以期为进一步研究独栖蜂与生态系统中环境因素的相互作用提供理论依据。
1 材料与方法
1.1 实验地点
本研究的实验地点位于中国东南部的江西省新岗山镇(117°54° E, 29°07° N)的亚热带森林中。实验样点包括A样地和B样地, 两个样地相距4 km, 面积共50 ha。在两个样地共设计了566个样方(每个样方大小为25.8 m × 25.8 m)。样方中所有树木均为本地物种(乔木物种信息请参见附录1), 分别于2009年(A样地)和2010年(B样地)种植(Bruelheide et al, 2014)。实验将两个样地原来的树木全部砍伐, 按照树种丰富度梯度重新栽种。每个样方种植树木400株, 共20行20列, 每棵树间距离1.29 m。两个样地共选择88个样方设置巢管, 其中A样地40个样方, B样地48个样方。A样地样方间平均距离为324.3 m, B样地样方间平均距离为314.2 m, 以确保覆盖整个样地(样方分布参见附录2)。88个样方由1种树(24个样方)、2种树(16个)、4种树(16个)、8种树(16个)、16种树(12个)和24种树(4个)的多样性梯度组成(树种信息参见附录3、4)。树种多样性梯度按照断棍式(stick breaking)设计, 使每个树种在所有的多样性梯度下都能出现。
1.2 实验方法与材料
在每个样方的中心(7 m × 7 m)沿东北、西南方向对角线端点放置两个巢管, 管口东西朝向。每个巢管由芦苇管、塑料管和木桩组成。芦苇管长度150‒200 mm, 直径范围为0.2‒2.5 cm, 塑料管直径约12.5 cm, 长度22 cm。芦苇管使用当地野生芦苇(Phragmites sp.), 烘干后制作备用。巢管装置固定在木桩上, 高度约1.5 m。本研究的样本收集分别于2015、2016、2018、2019、2020年的4‒10月的月底进行。通过观察巢口是否有黏土或树脂封口判断是否筑巢, 将筑巢的芦苇管加标签带回实验室处理。
在实验室中剖开巢管, 测量并初步记录以下信息: 巢管收集日期、巢管长度、巢管直径、筑巢材料、封口材料、寄主食物、巢室数量、寄主数量、寄主物种、是否寄生、寄生者物种、寄生者数量。将处理好的巢管加标签放入玻璃管中, 用棉花塞紧瓶口在室温下饲养、观察。以天为单位定期观察试管中筑巢蜂及其寄生类群羽化情况, 确认并记录以下信息: 寄主物种、性别、羽化时间和羽化数量, 以及寄生者物种和羽化时间。选取部分样品针插制成干制标本进行后续鉴定。
所有数据录入到Excel 2010中, 统计分析在R 4.1.2中执行, 使用ggplot2、dplyr和ggsci包作图。
2 结果
2.1 独栖蜂多样性
巢管中全部样本共计25,354头, 其中独栖蜂21,154头。巢管中的独栖蜂属于6科56种。其中, 传粉者是独栖蜜蜂, 并不包括泥蜂科和方头泥蜂科。尽管泥蜂科和方头泥蜂科在最新的分类系统上属于蜜蜂总科, 但在本研究中根据食性将其归为捕食者功能群。传粉者共5,623头, 占全部独栖蜂的26.58%, 共有2科12种。捕食者有15,531头, 占全部独栖蜂的73.4%, 是整个巢管系统的优势类群, 包括蜾蠃、蛛蜂、泥蜂和方头泥蜂4类, 共有4科44种。所有独栖蜂物种如表1所示。
表1 生物多样性‒生态系统功能实验(BEF-China)新岗山样地独栖蜂物种组成
Table 1
| 科 Family | 种 Species | 科 Family | 种 Species |
|---|---|---|---|
| 分舌蜂科 Colletidae | 叶舌蜂一种 Hylaeus sp. 1 | 四代佳盾蜾蠃 E. quadrifasciatus | |
| 切叶蜂科 Megachilidae | 黑孔蜂 Heriades sauteri | 墨体胸蜾蠃 Orancistrocerus aterrimus | |
| 脊跗拟孔蜂 Hoplitis carinotarsa | 黑胸蜾蠃 O. drewseni | ||
| 白斑切叶蜂 Megachile strupigera | 丽旁喙蜾蠃 Pararrhynchium ornatum | ||
| 丘切叶蜂 M. monticola | 倾秀蜾蠃 Pareumenes quadrispinosus | ||
| 拟丘切叶蜂 M. pseudomonticola | 泥蜂科 Sphecidae | 日本蓝泥蜂 Chalybion japonicum | |
| 窄切叶蜂 M. rixator | 黑等齿泥蜂 Isodontia nigella | ||
| 粗切叶蜂 M. sculpturalis | 驼腹壁泥蜂 Sceliphron deforme | ||
| 细切叶蜂 M. spissula | 方头泥蜂科 Crabronidae | Passaloecus insignis | |
| 双切叶蜂 M. dinura | 褐带豆短翅泥蜂 Pison atripenne | ||
| 壮壁蜂 Osmia taurus | Pisoxylon sp. 1 | ||
| Trachusa staabi | 双色短翅泥蜂 Trypoxylon bicolor | ||
| 胡蜂科 Vespidae | 中华异喙蜾蠃 Allorhynchium chinense | Trypoxylon schmiedeknechtii | |
| 黄缘蜾蠃 Anterhynchium flavomarginatum | 蛛蜂科 Pompilidae | 垦丁奥沟蛛蜂 Auplopus kuarensis | |
| 黑唇元蜾蠃 Discoelius nigridypeus | 奥沟蛛蜂属一种 Auplopus carbonarius | ||
| 元蜾蠃属一种 Discoelius sp. 1 | 奥沟蛛蜂属一种 Auplopus sp. 1 | ||
| 王氏元蜾蠃 Discoelius wangi | 奥沟蛛蜂属一种 Auplopus sp. 2 | ||
| 福建埃蜾蠃 Epsilon fujianensis | 奥沟蛛蜂属一种 Auplopus sp. 3 | ||
| 方蜾蠃 Eumenes quadratus | 奥沟蛛蜂属一种 Auplopus sp. 4 | ||
| Eumenes sillicus | 奥沟蛛蜂属一种 Auplopus sp. 5 | ||
| 蜾蠃属一种 Eumenes sp. 1 | 奥沟蛛蜂属一种 Auplopus sp. 6 | ||
| 蜾蠃亚科一种 Eumeninae sp. 1 | 奥沟蛛蜂属一种 Auplopus sp. 7 | ||
| 蜾蠃亚科一种 Eumeninae sp. 2 | 奥沟蛛蜂属一种 Auplopus sp. 9 | ||
| 蜾蠃亚科一种 Eumeninae sp. 3 | 奥沟蛛蜂属一种 Auplopus sp. 10 | ||
| 蜾蠃亚科一种 Eumeninae sp. 4 | 奥沟蛛蜂属一种 Auplopus sp. 11 | ||
| 蜾蠃亚科一种 Eumeninae sp. 5 | 奥沟蛛蜂属一种 Auplopus sp. 12 | ||
| 蜾蠃亚科一种 Eumeninae sp. 8 | Deuteragenia ossarium | ||
| 显佳盾蜾蠃 Euodynerus notatus | 蛛蜂科一种 Pompilidae sp. 1 |
2.2 独栖蜂寄生者多样性
寄生者包括3目19科72种。寄生者分为两类: 一类是寄生在独栖蜂中的拟寄生者和盗寄生者, 另一类是寄生在独栖蜂食物中的寄生者。食物寄生者是巢管里的一种特殊情况(不同于盗寄生者), 通常是寄生者先在猎物体内寄生, 然后猎物被蜾蠃等捕获成为食物。被寄生的猎物并不会被取食, 寄生者会在巢管内羽化, 但并不影响独栖蜂的正常羽化。
拟寄生者涉及3目: 鞘翅目、膜翅目和双翅目, 包括14科36种。盗寄生类群涉及2个目, 分别是膜翅目和双翅目, 包括4科15种。多胚跳小蜂属一种(Copidosoma sp. 1)的理论寄主是鳞翅目幼虫, 但观察发现整个巢室中蜾蠃和食物全部被取食, 同时多胚跳小蜂只有1头样本, 因此归为拟寄生者类群。全部寄生类群物种如表2所示。
表2 新岗山样地独栖蜂全部寄生类群物种组成
Table 2
| 科 Family | 种 Species |
|---|---|
| 膜翅目 Hymenoptera | |
| 茧蜂科 Braconidae | Braconidae sp. 1 |
| 姬小蜂科 Eulophidae | Chaenotetrastichus semiflavus |
| Eulophidae sp. 1 | |
| Kocourekia sp. 1 | |
| Melittobia australica | |
| M. clavicornis | |
| M. sosui Melittobia sp. 1 Melittobia sp. 2 | |
| 青蜂科 Chrysididae | 青蜂亚科一种 Chrysidinae sp. 1 |
| 青蜂亚科一种 Chrysidinae sp. 2 | |
| 青蜂亚科一种 Chrysidinae sp. 3 | |
| 青蜂亚科一种 Chrysidinae sp. 4 | |
| 青蜂亚科一种 Chrysidinae sp. 5 | |
| Chrysis principalis | |
| Chrysura sp. 1 | |
| 跳小蜂科 Encyrtidae | 多胚跳小蜂属一种 Copidosoma sp. 1 |
| 姬蜂科 Ichneumonidae | Dusona sp. 1 |
| 姬蜂科一种 Ichneumonidae sp. 1 姬蜂科一种 Ichneumonidae sp. 5 姬蜂科一种 Ichneumonidae sp. 6 姬蜂科一种 Ichneumonidae sp. 7 Picardiella melanoleuca | |
| 褶翅小蜂科 Leucospidae | 日本褶翅小蜂 Leucospis japonicas |
| 褶翅小蜂属一种 Leucospis sp. 1 | |
| 钩腹蜂科 Trigonalyidae | Lycogaster flavonigrata |
| 青翅狼钩腹蜂 Lycogaster violaceipennis | |
| 蚁蜂科 Mutillidae | 蚁蜂科一种 Mutillidae sp. 1 蚁蜂科一种 Mutillidae sp. 2 |
| 巨胸小蜂科 Perilampidae | 巨胸小蜂科一种 Perilampidae sp. 1 |
| 膜翅目 Hymenoptera | |
| 长尾小蜂科 Torymidae | 长尾小蜂科一种 Torymidae sp. 1 |
| 切叶蜂科 Megachilidae | 厚腹尖腹蜂 Coelioxys crassiventris |
| C. fenestrate | |
| 尖腹蜂属一种 Coelioxys sp. 1 | |
| 基赤腹蜂 Euaspis basalis | |
| 波赤腹蜂 E. polynesia | |
| 褶翅蜂科 Gasteruptiidae | 窄头褶翅蜂 Gasteruption corniculigerum |
| 双翅目 Diptera | |
| 蜂虻科 Bombyliidae | 岩蜂虻属 Anthrax sp. 1 |
| 蜂虻科一种 Bombyliidae sp. 1 | |
| 蜂虻科一种 Bombyliidae sp. 2 | |
| 蜂虻科一种 Bombyliidae sp. 3 | |
| 蚤蝇科 Phoridae | 蚤蝇科一种 Phoridae sp. 1 |
| 蚤蝇科一种Phoridae sp. 2 | |
| 麻蝇科 Sarcophagidae | 耳摩蜂麻蝇 Amobia auriceps |
| 摩蜂麻蝇属一种 Amobia sp. 1 | |
| 蜂麻蝇属一种 Miltogramma sp. 1 | |
| 蜂麻蝇属一种 Miltogramma sp. 2 | |
| 蜂麻蝇属一种 Miltogramma sp. 3 | |
| 赛蜂麻蝇属一种 Senotainia sp. 1 | |
| 鞘翅目 Coleoptera | |
| 芫菁科 Meloidae | Zonitis sp. 1 |
| 大花蚤科 Ripiphoridae | 大花蚤科一种 Ripiphoridae sp. 1 |
食物寄生者涉及2个目, 分别是膜翅目和双翅目, 共有6科21种。所有食物寄生的类群寄生行为均发生在雌性独栖蜂捕食之前, 而且这种情况只出现在捕食者类群中。被寄生的猎物只是被捕食者麻醉, 因此通常寄生者会在巢室中完成发育并羽化。而几乎不影响巢管独栖蜂的生长发育, 所以这些寄生者与寄主无直接关系, 只是独栖蜂雌性的捕食行为将其联系起来。食物寄生类群物种如表3所示。
表3 新岗山样地独栖蜂食物寄生类群物种组成
Table 3
| 科 Family | 种 Species |
|---|---|
| 膜翅目 Hymenoptera | |
| 茧蜂科 Braconidae | 脊茧蜂属一种 Aleiodes sp. 1 |
| 长绒茧蜂属一种 Dolichogenidea sp. 1 | |
| 小腹茧蜂属一种 Microgaster sp. 1 | |
| 小腹茧蜂属一种 Microgaster sp. 2 | |
| 小腹茧蜂属一种 Microgaster sp. 3 | |
| 小腹茧蜂亚科一种 Microgastrinae sp. 1 | |
| 小腹茧蜂亚科一种 Microgastrinae sp. 2 | |
| Yelicones belokobylskiji | |
| 姬蜂科 Ichneumonidae | Skeatia sp. 1 |
| Skeatia sp. 2 | |
| 广肩小蜂科 Eurytomidae | Eurytoma sp. 1 |
| 双翅目 Diptera | |
| 厕蝇科 Fanniidae | Fanniidae sp. 1 |
| 寄蝇科 Tachinidae | Argyrophylax sp. 1 |
| 十和田阿特寄蝇 Atylostoma towadensis | |
| Belvosia sp. 1 | |
| Cordyligaster sp. 1 | |
| Exoristinae sp. 1 | |
| 花截尾寄蝇 Nemorilla floralis | |
| Patelloa sp. 1 | |
| Senometopia sp. 1 | |
| 麻蝇科 Sarcophagidae | Sarcophaga albiceps |
2.3 生物学特性
2.3.1 独栖蜂
传粉者主要为分舌蜂和切叶蜂, 以花粉为食; 捕食者主要为蜾蠃、蛛蜂、泥蜂和方头泥蜂。其中蜾蠃以鳞翅目幼虫为食(占捕食性蜂食物的86.16%); 蛛蜂、部分泥蜂和部分方头泥蜂以蜘蛛为食(占12.53%); 一种方头泥蜂以蚜虫为食(占0.34%); 一种泥蜂以草螽为食(占0.97%) (食物组成见附录5)。
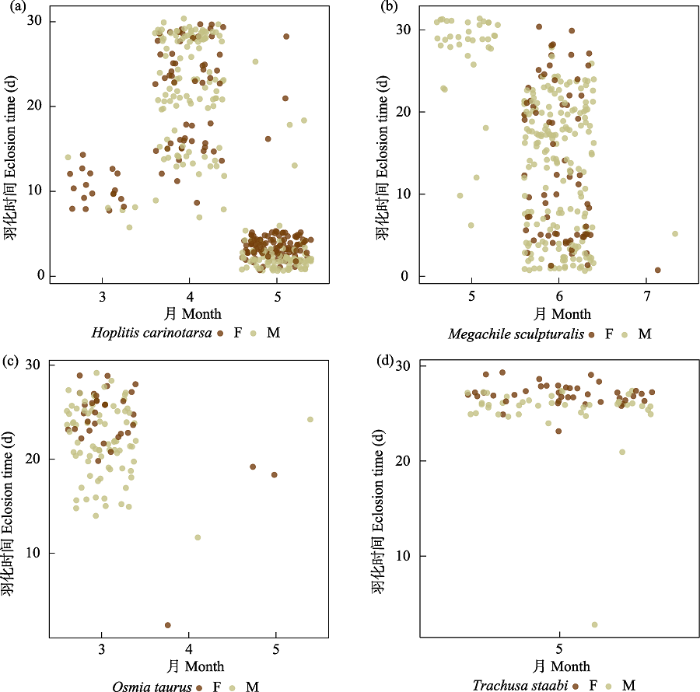
(1)分舌蜂科叶舌蜂。数量为125头, 在传粉者里比例很小, 约为2.2%。该蜂一年可发生多代, 筑巢活动时间是5‒9月, 羽化时间是4‒9月。其中5‒8月个体均在1个月左右羽化, 而9月份仅部分个体在1个月内羽化, 其余个体于次年4月底至5月初羽化。分舌蜂科物种的筑巢材料是自身的腺体分泌物, 形成膜质结构分割巢。窄头褶翅蜂专性盗寄生于该种分舌蜂, 羽化时间通常早于寄主分舌蜂。
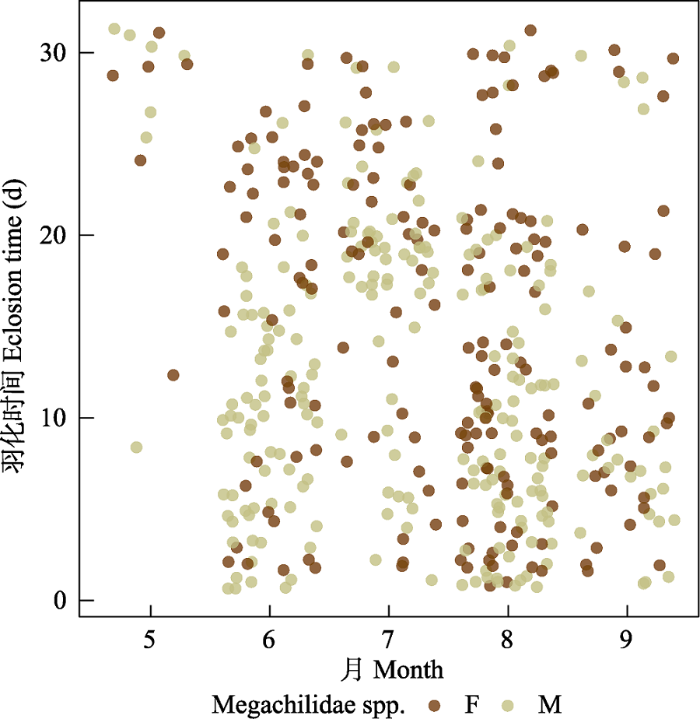
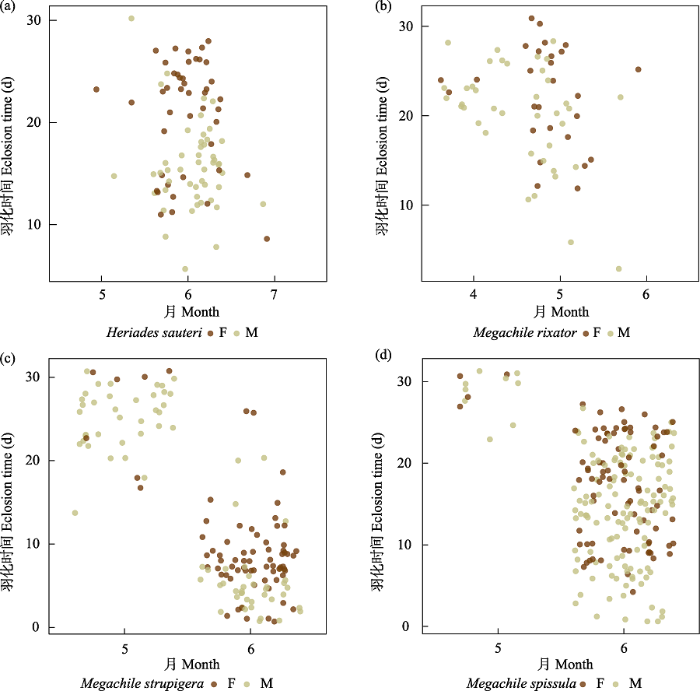
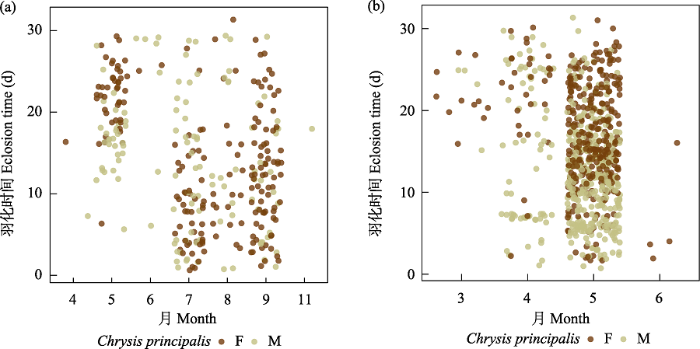
(2)切叶蜂。共5,498头, 占全部传粉者数量的97.8%, 是传粉的主要类群。切叶蜂共有11个种, 生活史相当多样化。双切叶蜂(Megachile dinura)、丘切叶蜂(M. monticola)和拟丘切叶蜂(M. pseudomonticola)是稀有种。其中双切叶蜂(占切叶蜂的0.21%)活动时间为9‒10月, 次年6月初越冬羽化; 丘切叶蜂(占切叶蜂的0.38%), 活动时间为7‒10月, 次年6月底至7月初羽化; 拟丘切叶蜂(占切叶蜂的0.43%), 活动时间为7‒9月, 9月底的个体则会在次年6月底羽化。黑孔蜂(Heriades sauteri)、窄切叶蜂(M. rixator)、白斑切叶蜂(M. strupigera)和细切叶蜂(M. spissula)是4种多化性切叶蜂, 活动时间为5‒10月, 5‒8月的个体会在1个月内完成发育(图1), 8月和9、10月的部分个体通常会越冬羽化, 不同类群越冬羽化时间略有差别(图2)。黑孔蜂(占切叶蜂的7.2%)会在次年6月中旬羽化; 窄切叶蜂(占6.6%), 白斑切叶蜂(占16.9%)次年4月中旬至5月中旬羽化; 细切叶蜂(占10%)次年5月底、6月初羽化(图2)。另外4种切叶蜂均是单化性, 一年一代, 幼虫期短, 多以预蛹越冬, 次年羽化, 羽化时间因种而异。脊跗拟孔蜂(Hoplitis carinotarsa) (占35.4%)活跃时间为5月, 于4月中旬至5月初羽化, 少数会在3月羽化; 粗切叶蜂(Megachile sculpturalis) (占8.9%)活动时间是5‒10月, 所有个体都会在越冬后于次年5月、6月羽化; 壮壁蜂(Osmia taurus) (占11.1%)活动时间为4月, 所有个体都在次年3月中旬至3月底羽化; Trachusa staabi (占2.8%)筑巢活动集中在6月, 所有个体会在次年5月底羽化(图3)。大部分切叶蜂不同性别之间羽化时间非常接近, 雄性比雌性早几天, 通常在1周以内, 而单化性蜜蜂的越冬个体雌雄羽化时间差异更显著。此外, 它们的筑巢材料相当多样化, 树叶、泥、树脂、木屑等是常用筑巢材料。这一点在粗切叶蜂中更明显, 同种的不同个体筑巢虽然主体形态相同, 但材料均多样化, 可能具有就近取材筑巢的习性。
图1
图1
4种多化性切叶蜂(黑孔蜂、窄切叶蜂、白斑切叶蜂和细切叶蜂)非越冬个体羽化时间图。F代表雌性, M代表雄性。
Fig. 1
Eclosion time of non-overwintering individuals of four multivoltine species of leaf cutting bees (Heriades sauteri, Megachile rixator, M. strupigera, M. spissula). F, Female; M, Male.
图2
图2
4种多化性切叶蜂越冬个体羽化时间图。(a)黑孔蜂; (b)窄切叶蜂; (c)白斑切叶蜂; (d)细切叶蜂。F代表雌性, M代表雄性。
Fig. 2
Eclosion time of overwintering individuals of four multivoltine species of leaf cutting bees. (a) Heriades sauteri; (b) Megachile rixator; (c) M. strupigera; (d) M. spissula. F, Female; M, Male.
图3
图3
4种单化性切叶蜂越冬个体羽化时间图。(a)脊跗拟孔蜂; (b)粗切叶蜂; (c)壮壁蜂; (d) Trachusa staabi。F代表雌性, M代表雄性。
Fig. 3
Eclosion time of overwintering individuals of four univoltine species of leaf cutting bees. (a) Hoplitis carinotarsa; (b) Megachile sculpturalis; (c) Osmia taurus; (d) Trachusa staabi. F, Female; M, Male.
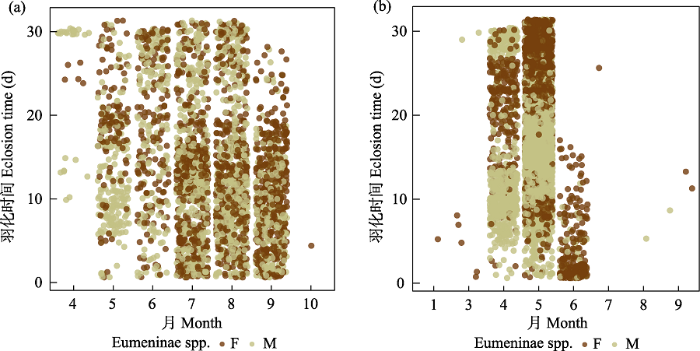
(3)蜾蠃。是巢管里最多的类群, 共计13,380头, 占所有捕食者的86.1%。蜾蠃一般一年发生4‒5代, 活动时间为5‒10月。8月的少数个体、9月的多数个体及10月全部个体会滞育越冬次年羽化; 其他月份个体均会在1个月内羽化。雄性羽化早于雌性, 时间从2天至2周左右不等。越冬羽化是在次年4‒6月初, 越冬个体雄性提前羽化现象更显著(图4)。黄缘蜾蠃(Anterhynchium flavomarginatum)是其中绝对的优势种, 在全部独栖蜂里占52.5%, 在全部捕食性蜂中占71.6%。其活动时间范围比其他蜾蠃更广, 4‒10月均有大量个体活动, 甚至是10月之后也有活动痕迹, 越冬个体次年4‒6月初羽化。其他数量较多的蜾蠃有3种, 黑胸蜾蠃(Orancistrocerus drewseni) (占全部蜾蠃的4.92%)在5‒10月活动, 9、10月的个体多在次年4月下旬至5月羽化; 福建埃蜾蠃(Epsilon fujianensis) (占4.32%)活动时间是5‒9月, 大部分同年1个月内羽化, 9月少数个体于次年4月中旬羽化; 倾秀蜾蠃(Pareumenes quadrispinosus) (占3.13%)集中活动时间是6‒9月, 8‒9月的个体于次年5月中旬至6月中旬羽化。大部分蜾蠃以泥土筑巢, 但是有时会在泥土上覆盖植物制成的纸状物质, 少数以纸状物、树脂或二者混合筑巢。蜾蠃通常捕猎鳞翅目幼虫为后代提供食物, 初步鉴定猎物大部分为鳞翅目夜蛾科物种。
图4
图4
捕食性蜾蠃羽化时间图。左为非越冬个体, 右为越冬个体。F代表雌性, M代表雄性
Fig. 4
Eclosion time of Eumeninae. Non-overwintering individuals on the left and overwintering individuals on the right. F, Female; M, Male.
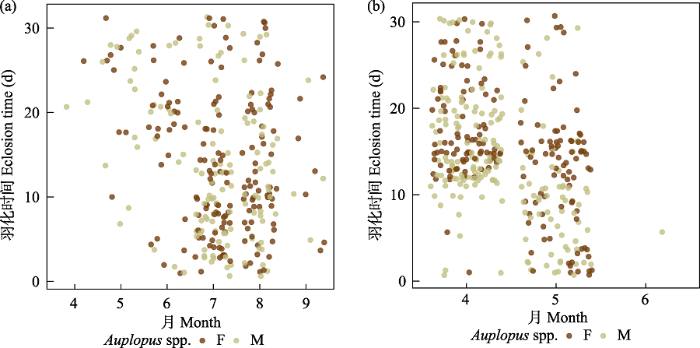
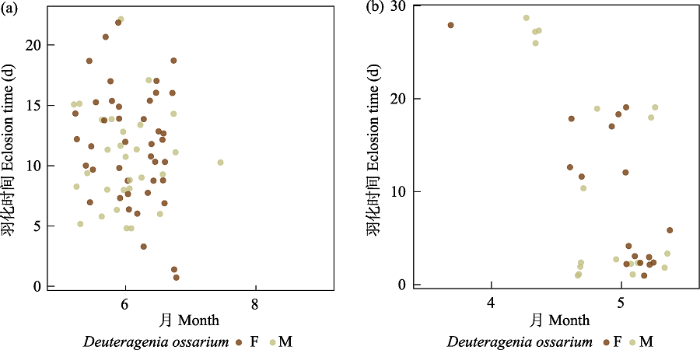
(4)蛛蜂。是巢管里比较常见的类群, 共有1,425头, 占全部捕食者的9.18%。主要有两类, 一类是奥沟蛛蜂属(Auplopus spp.)的物种, 占蛛蜂的87.7%;另一类是Deuteragenia ossarium, 占蛛蜂的12.3%。奥沟蛛蜂属的物种活动时间是4‒10月, 8月的部分个体及9月以后的个体全部滞育越冬, 于次年4月中旬至5月中旬羽化, 其余各月的个体均在1个月内羽化(图5)。D. ossarium的集中活动时间是在5月和6月上旬, 有时10月也能发现踪迹。推测该物种适宜温度比较低, 其中5月的个体在1个月内羽化, 而10月的个体会在次年4月底至5月上旬羽化(图6)。蛛蜂不同种虽然形态非常接近, 但是巢的形态非常多样化。筑巢材料主要是泥土, 少数物种使用其他材料, 如D. ossarium会将蚂蚁当成巢室的封口, 垦丁奥沟蛛蜂(Auplopus kuarensis)会将树脂当成封口材料。蛛蜂的捕食对象主要是蜘蛛, 奥沟蛛蜂属的物种均以跳蛛科蜘蛛为食, 而D. ossarium则是以漏斗蛛科蜘蛛为食。
图5
图5
蛛蜂科奥沟蛛蜂属羽化时间图。左为非越冬个体, 右为越冬个体。F代表雌性, M代表雄性。
Fig. 5
Eclosion time of genus Auplopus (Pompilidae). Non-overwintering individuals on the left and overwintering individuals on the right. F, Female; M, Male.
图6
图6
蛛蜂科Deuteragenia ossarium羽化时间图。左为非越冬个体, 右为越冬个体。F代表雌性, M代表雄性。
Fig. 6
Eclosion time of Deuteragenia ossarium (Pompilidae). Non-overwintering individuals on the left and overwintering individuals on the right. F, Female; M, Male.
(5)泥蜂和方头泥蜂的数量较少, 共计717头, 占捕食者的4.62%。不同物种的活动时间差异大: 日本蓝泥蜂(Chalybion japonicum)活动时间为6‒10月, 8月及以后的个体会于次年5月越冬羽化; 黑等齿泥蜂(Isodontia nigella)活动时间为4‒10月, 8月及以后的个体会越冬于次年5月羽化; 驼腹壁泥蜂(Sceliphron deforme)活动时间为4‒10月, 9月以后的多数个体于次年5月中旬至6月中羽化; Passaloecus insignis活动时间为5‒7月, 均在1个月内羽化, 褐带豆短翅泥蜂(Pison atripenne)活动时间5‒10月, 9、10月个体越冬羽化; Pisoxylon sp. 1活动时间为5月, 一个月内羽化; 双色短翅泥蜂(Trypoxylon bicolor)活动时间是 5‒9月, 9、10月的个体会在次年5月羽化; T. schmiedeknechtii活动时间是9月以后, 次年6月开始羽化。通常雄性早于雌性羽化, 约在1周以内, 但是黑等齿泥蜂雄性羽化时间显著早于雌性, 二者差距可以达2周时间。它们的捕食猎物范围极广, 可以涵盖多个目, 大部分泥蜂会捕猎蜘蛛哺育后代, 但是也有很多其他的猎物类型, 如褐带豆短翅泥蜂以蚜虫为猎物, 黑等齿泥蜂以草螽为猎物。
2.3.2 寄生者
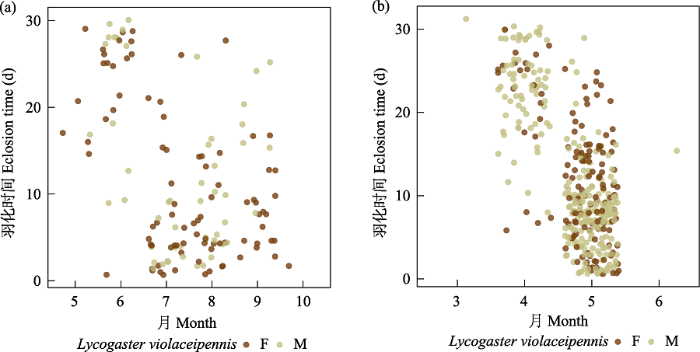
本研究中的寄生者是指与巢管中独栖蜂直接关联的拟寄生和盗寄生类群, 而不考虑通过捕食关联的食物寄生者。因为在一个寄主的巢室里孵化出多头寄生者, 所以本研究统计的寄生者数量是按照被寄生的寄主数量来计算的。寄生者的总数量为4,076头, 主要是双翅目的摩蜂麻蝇属(Amobia spp.)和岩蜂虻属一种(Anthrax sp. 1); 膜翅目的Chrysis principalis、青翅狼钩腹蜂(Lycogaster violaceipennis)、Melittobia sosui和尖腹蜂属(Coelioxys spp.) (表4)。
表4 独栖蜂主要寄生者多样性概况
Table 4
| 独栖蜂寄生者 Parasitoid | 数量 Number | 占比 Proportion |
|---|---|---|
| 岩蜂虻属一种 Anthrax sp. 1 | 127 | 3.12% |
| 摩蜂麻蝇属 Amobia spp. | 1,007 | 24.71% |
| Chrysis principalis | 1,244 | 30.52% |
| 尖腹蜂属 Coelioxys spp. | 128 | 3.14% |
| 青翅狼钩腹蜂 Lycogaster violaceipennis | 597 | 14.65% |
| Melittobia sosui | 490 | 12.02% |
| 其他 Other | 529 | 12.98% |
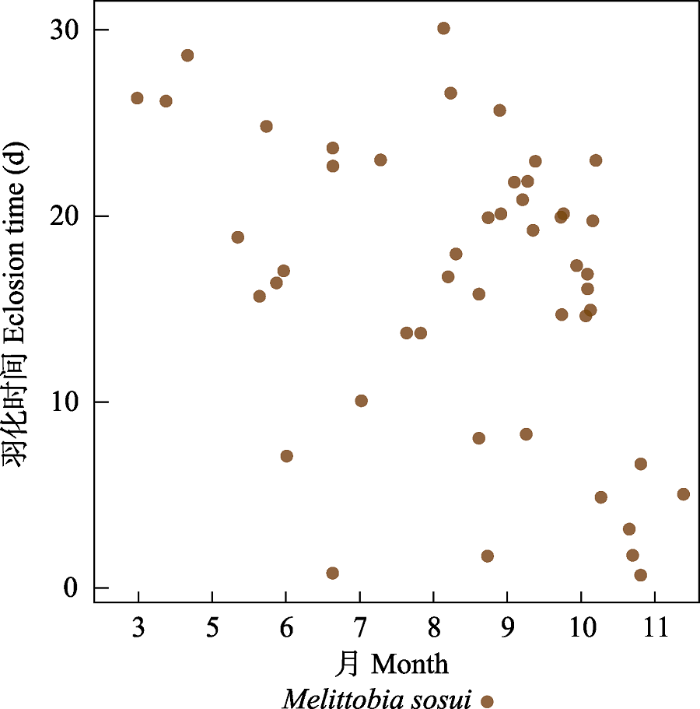
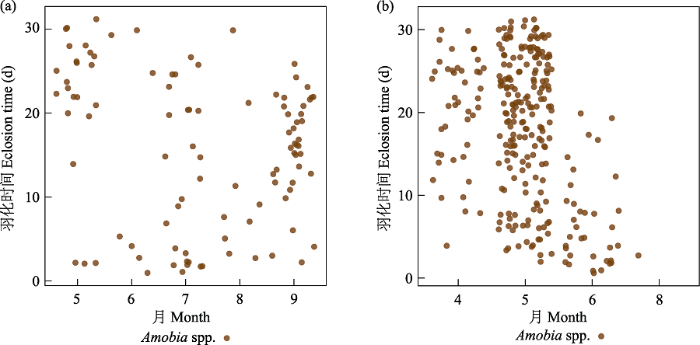
除了岩蜂虻属一种和Melittobia sosui的寄主范围相当广之外, 其他类群的寄生专化性较强。岩蜂虻属一种有127头, 占寄生者的3.11%, 寄主为5个科(胡蜂科蜾蠃亚科、泥蜂科、方头泥蜂科、切叶蜂科和蛛蜂科) 15个种。活动时间为6‒10月, 9月以后的越冬个体于次年5、6月羽化。M. sosui有444头, 占寄生者的10.9%, 其寄主为5个科(胡蜂科蜾蠃亚科、泥蜂科、方头泥蜂科、切叶蜂科和蛛蜂科) 28个种。Melittobia属的成员基本全年都可活动, 但很少越冬羽化, 大部分在同年羽化(图7)。摩蜂麻蝇属占寄生类群的24.7%, 主要是黄缘蜾蠃的盗寄生类群, 寄生于其他蜾蠃、泥蜂和蛛蜂的只发现了45例, 而只发现了1例寄生于白斑切叶蜂的情况。活跃时间为5‒10月, 9、10月的个体越冬于次年4月至6月中旬羽化(图8)。Chrysis principalis有1,244头, 占全部寄生者的30.5%, 专性寄生在黄缘蜾蠃上, 只有30头寄生在其他蜾蠃上。活跃时间为5‒10月, 9、10月的个体越冬并于次年4‒5月羽化(图9)。青翅狼钩腹蜂有597头, 占14.6%, 主要寄生于黄缘蜾蠃和黑胸蜾蠃,只发现9例寄生于其他蜾蠃。活跃时间为5‒10月, 9、10月的大部分个体于次年4‒5月羽化(图10)。尖腹蜂属有3个种, 其中厚腹尖腹蜂(Coelioxys crassiventris) 有75头, 占全部寄生者的1.84%, 只寄生在窄切叶蜂上; C. fenestrata有52头, 占全部寄生者的1.27%, 寄生于粗切叶蜂和双叶切叶蜂上; Coelioxys sp. 1只发现了1头, 寄生在丘切叶蜂上。尖腹蜂属很少同年羽化, 大部分个体都越冬在次年4月中旬至6月中旬羽化。这似乎和其寄主独栖蜂类群大都越冬羽化有关。几乎所有的寄生者都早于寄主羽化, 通常越冬个体在次年羽化, 但是Melittobia属可以不越冬而正常羽化。越冬的尖腹蜂、青蜂和钩腹蜂雄性通常早于雌性数天羽化, 而同年羽化的寄生者雌雄之间没有显著差异。
图7
图7
寄生者膜翅目姬小蜂科Melittobia sosui羽化时间图
Fig. 7
Elosion time of Melittobia sosui (Eulophidae), a parasitoid species
图8
图8
寄生者双翅目摩蜂麻蝇属羽化时间图。左为非越冬个体, 右为越冬个体。
Fig. 8
Eclosion time of genus Amobia (Sarcophagidae), a parasitoid species. Non-overwintering individuals on the left and overwintering individuals on the right
图9
图9
寄生者膜翅目青蜂科Chrysis principalis羽化时间图。左为非越冬个体, 右为越冬个体。F代表雌性, M代表雄性。
Fig. 9
Eclosion time of Chrysis principalis (Chrysididae). Non-overwintering individuals on the left and overwintering individuals on the right. F, Female; M, Male.
图10
图10
寄生者膜翅目青翅狼钩腹蜂羽化时间图。左为非越冬个体, 右为越冬个体。F代表雌性, M代表雄性。
Fig. 10
Eclosion time of Lycogaster violaceipennis (Trigonalyidae). Non-overwintering individuals on the left and overwintering individuals on the right. F, Female; M, Male.
3 讨论
3.1 独栖蜂多样性的组成差异
本研究表明, 在中国亚热带森林中传粉者多度占独栖蜂的26.6%, 捕食者占73.4%, 比例约为1 : 2.76,而物种丰富度比例约为1 : 3.6, 捕食蜂多样性远大于独栖蜜蜂。且捕食蜂筑巢的巢管数为11,232个,筑巢率约为84.6%; 传粉者筑巢的巢管数为2,035个,筑巢率约为15.4%。在巴西的热带森林, 捕食者筑巢率约为75% (Loyola & Martins, 2006), 与本研究筑巢率85%结果相近。这可能反映了自然环境中筑巢独栖蜂中传粉者和捕食者的真实组成。实际上, 筑巢的独栖蜜蜂类群较少, 且其中约64%的类群会在地下挖土筑巢(Cane & Neff, 2011), 剩下的大部分个体(主要是切叶蜂)会在天然空腔洞穴中筑巢(Danforth et al, 2019); 捕食蜂大多选择空腔筑巢(Evans, 1966)。有洞穴筑巢习性的传粉者和捕食者的不同比例可能直接导致了传粉者数量少于捕食者。此外, 多项研究已经证明, 独栖蜜蜂的多样性与开花植物的多样性密切相关(Ebeling et al, 2012), 与树木多样性关联性较小(Guo et al, 2021)。而本研究样地里高大乔木居多, 地面的开花植物少, 再加上每年除草, 人为去除地面开花植物; 同时独栖性蜜蜂与社会性蜂相比具有更窄的食性, 种群内个体间也缺少通讯能力, 不能进行远距离的觅食活动(Danforth et al, 2019)。所以本研究的样地环境实际上不利于独栖性蜜蜂生存, 导致传粉者数量少于捕食者。
3.2 独栖蜂羽化时间
雄性先羽化现象在昆虫中普遍存在, 如蝴蝶(Zonneveld & Metz, 1991)、二化螟(肖丹凤和胡阳, 2010)、蝗虫(del Castillo & Núñez-Farfán, 2002)等都曾记录到该现象。此外, 在某些膜翅目物种如方头泥蜂、切叶蜂科的物种中也观察到过该现象(Polidori et al, 2010)。本研究同样发现了筑巢独栖蜂明显的雄性先羽化现象: 独栖蜂中大部分都是雄性先羽化, 只有蛛蜂是雌雄几乎同时羽化。在筑巢产卵行为上, 独栖蜂表现出了对雄性先羽化行为的适应: 雌性可以控制产下后代的性别(雄性为单倍体, 雌性为二倍体), 在巢穴中呈线型排列; 雌性的巢在内侧, 而雄性的巢在外侧。这样避免了雌性阻挡先羽化的雄性出巢(Gerber & Klostermeyer, 1970)。对于雄性先羽化现象(Fagerstrom & Wiklund, 1982)有很多解释: (1)可以防止近亲繁殖(Petersen, 1892); (2)确保只有存活足够长时间的最高适合度雄性才能与雌性交配(Demoll, 1908); (3)确保雌性在羽化后立即受精以尽量减少其在生殖前的死亡(Lundgren & Bergström, 1975); (4)雄性先羽化比在雌性之后羽化有更多交配的机会(Petersen, 1947)。雄性先羽化类群有很多特征, 在高密度、雌雄二型(雌性更大)、雄性比重大和雌性只交配一次的昆虫种群中, 往往会出现雄性先羽化现象(del Castillo & Núñez-Farfán, 2002)。对蝴蝶的雄性先羽化行为研究认为, 该行为的维持主要取决于雌性只交配一次而雄性能够多次交配的机制(Zonneveld & Metz, 1991)。本研究中的雄性先羽化类群基本符合这些特征。蜾蠃、泥蜂和切叶蜂雌雄二型明显, 除一些形态差异外, 雌性个体明显大于雄性。蛛蜂雌雄个体大小相近, 最显著的差异特征为触角(雌性触角盘旋成漩涡状)。此外, 蜾蠃、泥蜂和切叶蜂性比更偏重雄性, 其中蜾蠃雄性雌性比约为1.64 : 1; 泥蜂约为1.97 : 1; 切叶蜂约为1.65 : 1。而蛛蜂雄性雌性比为0.97 : 1, 接近1 : 1。因此, 相较于蛛蜂, 蜾蠃、泥蜂和切叶蜂有雄性早于雌性的羽化现象, 符合预期。对切叶蜂属的研究认为雄性先羽化可以增加雌雄的交配成功率, 并减少雌性生殖前的空白期(Paini, 2004)。该解释可推广至其他独栖蜂类群, 在一定程度上解释了独栖蜂雄性先羽化现象。独栖蜂相比于社会性蜂活动时间更短, 一个典型的独栖蜂成虫活动时间仅仅为几个星期到1个月(Minckley et al, 1994), 独栖蜂必须在有限的时间内成功交配繁殖, 而雄性先羽化增加了交配成功率, 因此该策略对种群繁衍有重要意义。但在越冬个体中观察到异常明显的雄性先羽化现象仍无法准确解释, 需要进一步研究。
3.3 不同类群的发生规律
独栖蜂活动的季节性规律在很多地区的研究中都有记录(Camillo et al, 1996; Ribeiro & Garófalo, 2010), 如巴西亚热带地区的Podium denticulatum活动集中在炎热多雨的9月到次年4月(Shibata et al, 2020); 巴西亚热带的Megachile nigripennis主要在11月至次年2月活动(Marques & Gaglianone, 2013)。在北美洲亚热带的火炬松(Pinus taeda)林中对独栖蜂的季节活动规律的研究表明, 同一地区切叶蜂、蜾蠃、泥蜂和方头泥蜂筑巢活动规律显著不同, 2种切叶蜂活动时间集中在4月至5月中旬, 而8种捕食蜂活动时间集中在5月下旬至8月(Jenkins & Matthews, 2004)。本研究结合筑巢时间和羽化时间印证了独栖蜂活动具有季节性的结论(表5), 发现亚热带地区不同独栖蜂类群的发生规律具有差异。切叶蜂和方头泥蜂活动时间较集中, 甚至仅在单个月发生; 而蜾蠃和蛛蜂活动时间范围较广, 在全年多个月份都能发生。这种差异与环境温度直接相关, 有研究表明独栖蜂会在寒冷和干燥时期减少活动(Camillo et al, 1996; Camillo, 2001)。同时, 独栖蜂的羽化时间也受温度影响, 较高的温度能促进独栖蜂的羽化, 较低的温度则推迟羽化(Danforth et al, 2019)。但温度对独栖蜂的影响并不是简单的线性关系, 而是不同类群对温度的适应性不同, 有自己的生态幅。因此不同类群的活动时间有季节性规律。另一个影响独栖蜂活动时间的因素是食物资源的同步情况(Danforth et al, 2019)。独栖蜜蜂与开花植物密切相关, 传粉者的活动时间往往同步于植物花期。而捕食性蜂的食物资源没有严格的时间限制, 作为其食物的鳞翅目幼虫和蜘蛛全年可获得, 因此活动时间范围更广。这也是捕食者类群多样性更高的原因。
表5 新岗山样地主要类群筑巢、羽化时间
Table 5
| 筑巢时间 Nesting time | 羽化时间 Eclosion time | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 物种 Species | 4月 April | 5月 May | 6月 June | 7月 July | 8月 Aug. | 9月 Sept. | 10月 Oct. | 3月 March | 4月 April | 5月 May | 6月 June | 7月 July | 8月 Aug. | 9月 Sept. |
| Heriades sauteri | √ | √ | √ | √ | ||||||||||
| Hoplitis carinotarsa | √ | √ | √ | √ | ||||||||||
| Hylaeus sp. 1 | √ | √ | √ | √ | ||||||||||
| Megachile rixator | √ | √ | √ | √ | √ | |||||||||
| M. sculpturalis | √ | √ | √ | √ | √ | |||||||||
| M. spissula | √ | √ | √ | |||||||||||
| M. strupigera | √ | √ | √ | √ | √ | √ | √ | √ | ||||||
| Osmia taurus | √ | √ | √ | |||||||||||
| Trachusa staabi | √ | √ | ||||||||||||
| Anterhynchium flavomarginatum | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |
| Epsilon fujianensis | √ | √ | √ | √ | √ | √ | ||||||||
| Auplopus spp. | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||
| Deuteragenia ossarium | √ | √ | √ | √ | ||||||||||
| Isodontia nigella | √ | √ | √ | |||||||||||
| Pareumenes quadrispinosus | √ | √ | √ | √ | √ | √ | ||||||||
| Sceliphron deforme | √ | √ | √ | √ | √ | √ | √ | √ | √ | |||||
| Orancistrocerus drewseni | √ | √ | √ | √ | √ | √ | √ | |||||||
| Passaloecus insignis | √ | √ | √ | |||||||||||
| Pisoxylon sp. 1 | √ | √ | √ | √ | ||||||||||
| Pison atripenne | √ | √ | √ | √ | √ | |||||||||
| Trypoxylon bicolor | √ | √ | √ | √ | √ | |||||||||
| T. schmiedeknechtii | √ | √ | ||||||||||||
表中物种的中文名称参见
All Chinese names refer to
3.4 寄生者与独栖蜂互作
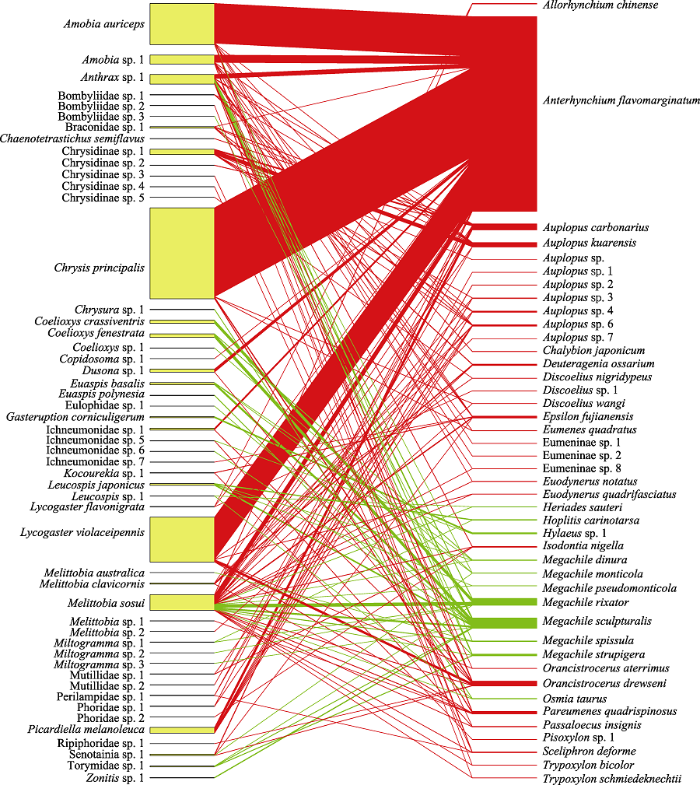
据上行控制效应, 高营养级生物受较低营养级生物的影响。自下而上的营养级联表明, 植物物种丰富度的增加可导致食草动物和捕食者物种丰富度的增加(Knops et al, 1999)。相似的研究也表明较高的植物多度可以支持植食性动物的高多度, 进而支持捕食者的高多度(Hunter & Price, 1992)。研究发现寄生者(高营养级)的多度和多样性能受到寄主(低营养级)上行控制效应的调节。独栖蜂和寄生者互作网络(图11)表明多度最高的黄缘蜾蠃其寄生者物种丰富度最高(多达14种), 这符合上行控制效应。同时寄生者Chrysis principalis和摩蜂麻蝇属有着较高的多度, 其主要寄主是黄缘蜾蠃, 这也与上行效应控制一致。此外, 寄生范围最广的寄生蜂Melittobia sosui多度也远高于其他寄生者物种, 而寄生范围相对较窄的寄生蜂, 如Euaspis basalis, Picardiella melanoleuca, Gasteruption corniculigerum等, 其多度较小, 这表明寄生者的数量也与其寄生范围的专化程度有关系。
图11
图11
独栖蜂与寄生者互作关系图。左边矩形代表寄生者, 右边矩形代表独栖蜂, 面积大小代表该物种互作的多度。物种中文名可参考
Fig. 11
The diagram of interaction between cavity-nesting Hymenoptera and parasitoids. The rectangle on the left represents parasitoids; the rectangle on the right represents cavity-nesting Hymenoptera; the size of rectangular area represents the abundance of species interaction. All Chinese names refer to
综上, 本文基于巢管法, 通过长期监测揭示了亚热带森林不同功能群独栖蜂及寄生者的多样性和生物学特性。独栖蜂中传粉者多样性显著少于捕食者(多度比例约1 : 3, 物种丰富度比例为1 : 3.7)。独栖蜂中普遍存在雌雄羽化异律的现象, 主要表现为雄性先羽化, 并在越冬个体中更明显。据推测这是为了防止近亲交配和减少雌性在生殖前的死亡。我们总结了不同类群的发生规律, 其中以切叶蜂和方头泥蜂为主的独栖蜂发生时间比以蜾蠃和蛛蜂为主的捕食蜂发生时间更集中。此外, 通过构建独栖蜂和寄生者互作关系, 发现寄生者的多度和多样性受较低营养级寄主的上行控制效应调节。
附录 Supplementary Material
附录1 BEF-China树种丰富度实验样地树种组成
Appendix 1 Tree species composition in BEF-China tree diversity experiment
附录2 新岗山样地巢管样方分布图
Appendix 2 The distribution of trap nests in Xingangshan plots
附录3 A样地巢管设置样方树种组成
Appendix 3 Tree species composition of trap nest plots in Site A
附录4 B样地巢管设置样方树种组成
Appendix 4 Tree species composition of trap nest plots in Site B
附录5 新岗山样地独栖蜂食物图
Appendix 5 The food of cavity-nesting Hymenoptera in Xingangshan plots
致谢
感谢中国科学院动物研究所袁峰对物种鉴定提供帮助, 吴清涛对实验提供大力支持; 感谢齐银泉师傅等对样本收集以及一同在新岗山野外采样团队的帮助。
参考文献
Nesting biology of Isodontia diodon (Kohl, 1890) (Hymenoptera: Sphecidae), a predator of cockroaches, in Hong Kong
Trap-nesting Ancistrocerus sikhimensis (Hymenoptera: Eumenidae) in Nepal: Nest structure and associates (Hymenoptera: Chrysididae; Acarina: Saproglyphidae)
DOI:10.1653/0015-4040(2005)088[0135:TASHEI]2.0.CO;2 URL [本文引用: 1]
Designing forest biodiversity experiments: General considerations illustrated by a new large experiment in subtropical China
DOI:10.1111/mee3.2014.5.issue-1 URL [本文引用: 2]
Nesting biology of Podium angustifrons Kohl (Hymenoptera, Sphecidae) in an Araucaria forest fragment
DOI:S1519-69842014000200493
PMID:25166337
[本文引用: 1]

Podium angustifrons Kohl 1902 is a species of solitary wasp which nests in pre-existing cavities, with neotropical distribution in Argentina, Bolivia, Brazil, Colombia, Guyana and French Guyana. The aim of this study was to investigate the nesting biology of P. angustifrons, discussing aspects of their life history. To capture its nests, wooden trap-nests were installed in the Parque Municipal das Araucárias, Guarapuava (PR), Brazil, from January 2003 to April 2009. A total of 29 nests were collected, all during the warmer months. These showed no vestibular and intercalary cells, and their closures were made up of chewed plants and mud mixed with organic materials and resin-coated surfaces, sometimes showing a layer of lichens. The cells were provisioned with various wild species of cockroaches (Chorisoneura sp, Riata sp and Helgaia sp) in the nymph stage and/or adults. The sex ratio was 4.6 females per male, significantly higher that the expected 1:1. Most pre-pupae entered diapause in winter with development time ranging from 187 to 283 days for females and 180 to 283 days for males. Deaths occurred in 41.66% of cells provisioned, 33.33% were attributed to faulty development and 8.33% to Chrysididae.
Inquilines of Brachymenes dyscherus nests with special reference to Monobia schrottkyi (Hymenoptera, Vespidae, Sphecidae)
Sixty-four inactive nests of the solitary mud-daubing wasp Brachymenes dyscherus, reused by 5 inquiline species, were collected at Fazenda Santa Carlota, Cajuru, São Paulo, Brazil in 1995 and 1996. Monobia schrottkyi used 52 nests; among the 717 cells available for use, 502 were reused. The number of cells per nest varied from 3 to 24; 1 to 16 individuals emerged from September to April (154 males and 112 females). Forty-six cells were parasitized by Melittobia sp. (n = 44) and Ichneumonidae (n = 2). Monobia curvata used 3 nests; among the 50 cells available for use, 38 were reused and 15 males and 8 females emerged from August to November. Three cells were parasitized by Ichneumonidae. Montezumia petiolata occupied 1 nest; among the 8 available cells, 7 were reused and 2 males and 3 females emerged in September. Podium denticulatum used 2 nests; the 20 cells available for use were reused and 11 males and 4 females emerged in August. Trypoxylon rogenhoferi used 5 nests that had 65 available cells; 48 of them were reused, from which 19 males and 11 females emerged from September to November. Three cells were parasitized by Ichneumonidae (n = 2) and Chrysididae (n = 1).
Substrates and materials used for nesting by North American Osmia bees (Hymenoptera: Apiformes: Megachilidae)
DOI:10.1603/0013-8746(2007)100[350:SAMUFN]2.0.CO;2 URL [本文引用: 1]
Predicted fates of ground-nesting bees in soil heated by wildfire: Thermal tolerances of life stages and a survey of nesting depths
DOI:10.1016/j.biocon.2011.07.019 URL [本文引用: 1]
Biology of a trap-nesting wasp of one species the ground-nesting Liris (Hymenoptera: Crabronidae) from the Atlantic Forest of southern Brazil
Nesting biology of Pareumenes quadrispinosus (de Saussure, 1855) (Hymenoptera: Vespidae: Eumeninae) in trap nests in North Vietnam
DOI:10.1016/j.aspen.2021.08.020 URL [本文引用: 1]
Nesting biology of the potter wasp Rhynchium brunneum brunneum (Fabricius, 1793) (Hymenoptera: Vespidae: Eumeninae) in north Vietnam
DOI:10.1016/j.aspen.2019.02.003
[本文引用: 1]

Rhynchium brunneum brunneum (Fabricius, 1793) is a common species using trap nests in North Vietnam. The females chose the nest traps with diameters ranging from 5.5 to 17 mm. Nests consisted of a linear series of one to eleven brood cells separated by mud partitions. Brood cells were provisioned with caterpillars, and eggs were attached to the ceiling of the cells by a thin filament. The life history and sex ratio data of this species were recorded from April to early November. Its sex ratio is strongly male-biased, being multivoltine, likely with four generations per year, the last one overwintering in the prepupal stage. Nesting activity of the species was described with major activities such as nesting site selection, oviposition, prey collecting, and applying cell material. Only 53.3% of the provisioned cells were successful; the others were damaged by six parasitoid species or died during development for unknown reasons.
Female mating success and risk of pre-reproductive death in a protandrous grasshopper
DOI:10.1034/j.1600-0706.2002.960203.x URL [本文引用: 2]
Die Bedeutung der Proterandrie bei Insekten
Effects of altitude gradient changes on nesting and biology for the potter wasp Anterhynchium flavomarginatum (Hymenoptera: Vespidae: Eumeninae)
海拔梯度变化对黄缘蜾蠃筑巢结构及相关生物学特性的影响
Multitrophic effects of experimental changes in plant diversity on cavity-nesting bees, wasps, and their parasitoids
DOI:10.1007/s00442-011-2205-8
PMID:22120706
[本文引用: 2]

Plant diversity changes can impact the abundance, diversity, and functioning of species at higher trophic levels. We used an experimental gradient in grassland plant diversity ranging from 1 to 16 plant species to study multitrophic interactions among plants, cavity-nesting bees and wasps, and their natural enemies, and analysed brood cell density, insect diversity (species richness), and bee and wasp community similarity over two consecutive years. The bee and wasp communities were more similar among the high (16 species) diversity plots than among plots of the lower diversity levels (up to 8 species), and a more similar community of bees and wasps resulted in a more similar community of their parasitoids. Plant diversity, which was closely related to flower diversity, positively and indirectly affected bee diversity and the diversity of their parasitoids via increasing brood cell density of bees. Increasing plant diversity directly led to higher wasp diversity. Parasitism rates of bees and wasps (hosts) were not affected by plant diversity, but increased with the diversity of their respective parasitoids. Decreases in parasitism rates of bees arose from increasing brood cell density of bees (hosts), whereas decreasing parasitism rates of wasps arose from increasing wasp diversity (hosts). In conclusion, decreases in plant diversity propagated through different trophic levels: from plants to insect hosts to their parasitoids, decreasing density and diversity. The positive relationship between plant diversity and the community similarity of higher trophic levels indicates a community-stabilising effect of high plant diversity.
The behavior patterns of solitary wasps
DOI:10.1146/ento.1966.11.issue-1 URL [本文引用: 1]
Why do males emerge before females? Protandry as a mating strategy in male and female butterflies
DOI:10.1007/BF00363830
PMID:28310501
[本文引用: 1]

The reproductive strategy of butterfly males can be defined as being to maximize the number of females mated. We have earlier shown that, if the eclosion period of females is regarded as given, males should emerge before females to achieve maximal reproductive success. However, females may also be considered to have a reproductive strategy with respect to the issue "when to emerge". In this paper we assume that females are selected to minimize the time spent unmated (to minimize prereproductive death), and analyze when females should optimally emerge in relation to males to achieve this end. We show that there is no conflict between the sexes with respect to the timing of eclosion when the length of the eclosion period is approximately equal for males and females. Thus, protandry should be considered a reproductive strategy of both males and females.
Multi-trophic communities re-establish with canopy cover and microclimate in a subtropical forest biodiversity experiment
DOI:10.1007/s00442-021-04921-y
[本文引用: 1]

Plant diversity affects multi-trophic communities, but in young regrowth forests, where forest insects are in the process of re-establishment, other biotic and also abiotic factors might be more important. We studied cavity-nesting bees, wasps and their natural enemies along an experimental tree diversity gradient in subtropical South-East China. We compared insect communities of experimental young forests with communities of established natural forests nearby the experiment and tested for direct and indirect effects of tree diversity, tree basal area (a proxy of tree biomass), canopy cover and microclimate on bee and wasp community composition, abundance and species richness. Finally, we tested if the trophic levels of bees, herbivore-hunting wasps, spider-hunting wasps and their natural enemies respond similarly. Forest bee and wasp community composition re-established towards communities of the natural forest with increasing tree biomass and canopy cover. These factors directly and indirectly, via microclimatic conditions, increased the abundance of bees, wasps and their natural enemies. While bee and wasp species richness increased with abundance and both were not related to tree diversity, abundance increased directly with canopy cover, mediated by tree biomass. Abundance of natural enemies increased with host (bee and wasp) abundance irrespective of their trophic position. In conclusion, although maximizing tree diversity is an important goal of reforestation and forest conservation, rapid closure of canopies is also important for re-establishing communities of forest bees, wasps and their natural enemies.
Sex control by bees: A voluntary act of egg fertilization during oviposition
The alfalfa leaf-cutter bee, Megachile rotundata, stops abdominal contractions briefly during oviposition of female eggs but not during oviposition of male eggs. Sperm stored in the spermatheca probably is pumped onto the micropyle of the egg during this pause. The stimulus inducing fertilization seems to be associated with the depth of the nesting tunnel.
A survey of the bees of the Black Rock Forest Preserve, New York (Hymenoptera: Apoidea)
Biological characteristics of Hylaeus perforata (Hymenoptera: Colletidae) and its natural enemy Gasteruption corniculigerum (Hymenoptera: Gasteruptiidae) and their correlation with environmental variables
缘叶舌蜂与其天敌窄头褶翅蜂的生物学特性及其与环境变量的相关性
Tree diversity promotes predatory wasps and parasitoids but not pollinator bees in a subtropical experimental forest
DOI:10.1016/j.baae.2021.03.007 URL [本文引用: 1]
On the nesting biology of Pirhosigma giordani soika (Hymenoptera, Vespidae, Eumeninae), with special reference to the use of vegetable matter
DOI:10.1590/S0085-56262013005000044 URL [本文引用: 1]
Bionomics of Euodynerus nipanicus (Schulthess)(Hymenoptera: Vespidae)
日本佳盾蜾蠃的生物学特性观察
Playing chutes and ladders: Heterogeneity and the relative roles of bottom-up and top-down forces in natural communities
DOI:10.1002/ecy.1992.73.issue-3 URL [本文引用: 1]
Evolution of instinct: Comparative ethology of Hymenoptera
Cavity-nesting Hymenoptera in disturbed habitats of Georgia and South Carolina: Nest architecture and seasonal occurrence
DOI:10.2317/0212.18a.1 URL [本文引用: 1]
Effects of plant species richness on invasion dynamics, disease outbreaks, insect abundances and diversity
DOI:10.1046/j.1461-0248.1999.00083.x
PMID:33810630
[本文引用: 1]

Declining biodiversity represents one of the most dramatic and irreversible aspects of anthropogenic global change, yet the ecological implications of this change are poorly understood. Recent studies have shown that biodiversity loss of basal species, such as autotrophs or plants, affects fundamental ecosystem processes such as nutrient dynamics and autotrophic production. Ecological theory predicts that changes induced by the loss of biodiversity at the base of an ecosystem should impact the entire system. Here we show that experimental reductions in grassland plant richness increase ecosystem vulnerability to invasions by plant species, enhance the spread of plant fungal diseases, and alter the richness and structure of insect communities. These results suggest that the loss of basal species may have profound effects on the integrity and functioning of ecosystems.Blackwell Science Ltd.
Nesting biology of Megachile (Eutricharea) studiosa Bingham, a leafcutter bee
DOI:10.1080/00218839.2019.1701776 URL [本文引用: 1]
Tusked males, male dimorphism and nesting behavior in a subsocial afrotropical wasp, Synagris cornuta, and weapons and dimorphism in the genus (Hymenoptera: Vespidae: Eumeninae)
DOI:10.2317/E-38.1 URL [本文引用: 1]
Trap-nest occupation by solitary wasps and bees (Hymenoptera: Aculeata) in a forest urban remanent
Temporal variation of solitary wasps and bees, nesting frequency, mortality, and parasitism were recorded from a remanent forest in Belo Horizonte, MG, Brazil. Wasps and bees were collected in trap-nests placed in areas with 25, 100, and 400 m2, from February to November 2004. The 137 trap-nests collected contained 11 species of wasps and bees. Wasps occupied most nests (75%). Occupation peaks occurred in March (25%) and September (26%); in June, the lowest occupation (2%) was observed. Except for Trypoxylon (Trypargilum) lactitarse Saussure, no significant correlation was found between number of occupied nests, and temperature and rainfall means. In the nests, 48% of the immature specimens died; 13% of the nests were parasitized. Total death and parasitism rates of wasps and bees differed significantly.
Wing scents and scent-released phases in the courtship behavior of Lycaeides argyrognomon (Lepidoptera: Lycaenidae)
DOI:10.1007/BF00988581 URL [本文引用: 1]
Nesting biology of three Megachile (Hymenoptera: Megachilidae) species from Eastern Amazonia, Brazil
Nesting biology and altitudinal variation in the abundance of Megachile (Melanosarus) nigripennis Spinolla (Hymenoptera, Megachilidae) in an Inselberg in the Mata Atlantica, Rio de Janeiro
Climate and food resources shape species richness and trophic interactions of cavity-nesting Hymenoptera
DOI:10.1111/jbi.v47.4 URL [本文引用: 2]
Biology of Ancistrocerus antilope (Panzer) (Hymenoptera, Vespidae) in trap-nests in Wisconsin
DOI:10.1093/aesa/49.1.97 URL [本文引用: 1]
Behavior and phenology of a specialist bee (Dieunomia) and sunflower (Helianthus) pollen availability
DOI:10.2307/1937464 URL [本文引用: 1]
Diversity of wild bees in wet meadows: Implications for conservation
DOI:10.1672/08-83.1 URL [本文引用: 1]
Local and landscape effects in a host-parasitoid interaction network along a forest-cropland gradient
DOI:10.1890/14-2476.1 URL [本文引用: 1]
Nesting biology of an Australian resin bee (Megachile sp.; Hymenoptera: Megachilidae): A study using trap nests
DOI:10.1111/aen.2004.43.issue-1 URL [本文引用: 2]
Die geographische Variation einiger Fennoskandischer Lepidopteren
Uber die Ungleichzeitigkeit in der Erscheinung der Geschlechter bei Schmentterlingen. Zoologische Jahrbücher
Emergence and dispersal relative to natal nest in the digger wasp Stizus continuus (Hymenoptera: Crabronidae)
DOI:10.1016/j.crvi.2009.11.014
PMID:20338545
[本文引用: 2]

The position of the emerging point has rarely been investigated as a factor possibly affecting the future nest settlement behaviour in Hymenoptera, in particular within nest aggregations. We studied the emergence and dispersion patterns of the digger wasp Stizus continuus. Individuals emerged daily in clumped patterns, possibly revealing a certain synchrony of emergence from the same nests, and protandry appeared both at seasonal and daily level. Differences between the number of females that nested relatively close or far from their emergence holes (EH) were either significant or not, depending on the year, and observed dispersal distances from the natal nests did not differ from those obtained by random simulations. By contrast, females nested close to the nearest conspecific nest. Size did not affect the dispersion patterns. EH are thus not important cues for nest establishment, and conspecific nests are probably the key cue for nest-founding females. In addition, males did not prefer to establish territories close to their natal nest.Copyright 2009 Académie des sciences. Published by Elsevier SAS. All rights reserved.
Nesting behavior of Podium denticulatum Smith (Hymenoptera: Sphecidae)
DOI:S1519-566X2010000600006
PMID:21271053
[本文引用: 1]

The nesting behavior of Podium denticulatum Smith was studied on the campus of Ribeirão Preto of the Universidade de São Paulo, SP, Brazil, from September 2003 to August 2005. The wasps established their nests in bamboo canes ranging from 11.4 cm to 26.2 cm in length and from 0.7 cm to 1.8 cm in internal diameter. Podium denticulatum nested almost exclusively in the hot and wet season (September-April), producing at least five generations per year. The cell provisioning was made with adult and nymphal cockroaches (Blattellidae) which were arranged venter-up and with the head inward toward the inner end of the cell. The construction of a temporary closure occurred in cells that took more than one day to be provisioned. The cells provisioned with a greater number of prey were more likely to produce females than males. The nests included 1-6 brood cells separated by mud partitions and arranged in a linear series. The innermost cells of the nests produced females, and the outermost cells produced males. Nests were parasitized by Eulophidae (Melittobia sp.), Chrysididae and Tachinidae.
The entomological evidence
DOI:10.1520/JFS14136J URL [本文引用: 1]
Nesting biology, sexual dimorphism, and populational morphometric variation in Podium denticulatum F. Smith, 1856 (Hymenoptera: Sphecidae)
DOI:10.13102/sociobiology.v67i4 URL [本文引用: 1]
Canopy vs. understory: Does tree diversity affect bee and wasp communities and their natural enemies across forest strata?
DOI:10.1016/j.foreco.2009.04.026 URL [本文引用: 1]
Trap nests for bees and wasps to analyse trophic interactions in changing environments—A systematic overview and user guide
DOI:10.1111/mee3.2018.9.issue-11 URL [本文引用: 2]
Bioindication using trap-nesting bees and wasps and their natural enemies: Community structure and interactions
DOI:10.1046/j.1365-2664.1998.355343.x URL [本文引用: 1]
Eastern carpenter bee (Hymenoptera: Apidae): Nest structure, nest cell provisions, and trap nest acceptance in Rhode Island
DOI:10.1093/ee/nvz032
PMID:30980666
[本文引用: 1]

Analysis of pollen provisions in Xylocopa virginica (L.) nests in southern Rhode Island showed that this species produced pollen loaves from 21 different genera of plants in 2016, 19 in 2017, and 39 in 2018. Antirrhinium majus L. (garden snapdragon) pollen was the most common type collected in all three years (21.4%). Overall, wind-pollinated tree pollen comprised 22.1% of all pollen loaves. Blueberry pollen was a minor component of pollen loaves (0.1%), despite abundant blueberry plants nearby. Mean values of X. virginica nest measurements (tunnel length 15.4 ± 1.2 cm, width 15.0 ± 0.5 mm, and cell length 17.7 ± 0.3 mm) were similar to those reported in previous studies. Only 2 of the 216 trap nests deployed in 2017 were occupied by 11 X. virginica bees (9 females and 2 males). However, 17 nests contained 230 Osmia taurus Smith, 6 nests contained 73 O. cornifrons (Radoszkowski), and 1 nest contained 8 O. lignaria Say. Thirty-four nests (15.7%) were occupied by 151 grass-carrying wasps, Isodontia sp. and 6 vespid wasps occupied three nests (1.4%) in 2017. In 2018, 4 of 96 trap nests were occupied by carpenter bees. Understanding the nesting and foraging habits of X. virginica will help us to manage natural populations for pollination services.© The Author(s) 2019. Published by Oxford University Press on behalf of Entomological Society of America. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
A preliminary research on protandry in rice striped stem borer
二化螟成虫雄性先羽化现象
Models on butterfly protandry: Virgin females are at risk to die
Current models on protandry in butterflies assume that females are mated instantaneously upon eclosion. However, for most butterfly species this assumption is not realistic. In this paper a model is formulated in which the mating rate depends on both male and female density. Given the female presence curve, protandry is an evolutionarily stable strategy (ESS) for males. The evolutionarily stable amount of protandry decreases with increasing death rate and decreasing encounter rate. Given the male presence curve, protandry also is an ESS for females. However, male and female ESS are not identical; moreover, in the present model a simultaneous ESS does not exist. Protandry critically depends on the assumption that females mate only once, whereas males are capable of multiple mating. If females too are capable of multiple mating, absence of protandry is the ESS for males as well as females. The model predicts that protandry depends on population density: protandry should be more pronounced in populations with high density than in populations with low density. Protandry also depends on sex ratio. It becomes more pronounced when the proportion of males among emerging adults increases.