物种是进化的基本单元(Simpson, 1951), 是研究生物多样性形成、演化和维持机制的重要内容(马克平, 1993), 同时也是生物多样性保护和可持续利用事业的基础(洪德元, 2016)。因此, 物种问题逐渐被生物学家所关注(孔宏智, 2016; 刘建全, 2016; 杨亲二, 2016; 葛颂, 2017), 而客观地认识以及划分物种是解决物种问题的重要前提。一般来说, 分类学研究主要包括性状的比较分析、归类和命名等三方面内容(方伟和刘恩德, 2012)。然而, 在自然界中, 植物易受环境因素和遗传因素影响从而产生表型性状的变化(Pigliucci et al, 2006; Leimar, 2009; 王姝和周道玮, 2017)。表型性状变化对分类学的比较和归类产生了很大困扰, 这种困扰的实质是对表型性状的变化规律认识不够深入和全面。所以, 在分类学研究中, 对争议物种进行群体水平大样本量的表型性状分析, 厘清表型性状变化式样, 明确物种间的真实分化状态, 是解决比较和归类等困扰的关键。
兰科石斛属(Dendrobium)植物为多年生草本,约1,450种, 主要分布于亚洲和大洋洲热带及其周边地区, 具有重要的观赏和药用价值(Cribb & Govaerts, 2005; Wood, 2006; Ng et al, 2012; Pridgeon et al, 2014)。石斛属物种繁多, 形态相似(Jin et al, 2009), 在种内和种间存在表型性状的重叠和间隔(Morris et al, 1996; Yukawa & Uehara, 1996; Adams, 2011), 这为研究物种分类和物种形成等问题提供了合适的研究体系。霍山石斛(D. huoshanense)为我国特有种(吉占和, 1980), 与河南石斛(D. henanense)和细茎石斛(D. moniliforme)等近缘种同属细茎石斛复合群(Jin et al, 2009; Xiang et al, 2013)。在这个复合群内霍山石斛与河南石斛的系统发育关系最近(徐晴, 2015)。前期研究与野外调查发现, 霍山石斛种内存在相当程度的表型性状变异, 模糊了物种界限, 造成了与其他近缘种的混淆, 进而严重影响了物种资源的保护和利用等各个方面。因此, 亟待从表型性状层面开展霍山石斛的比较和归类研究。
目前, 关于霍山石斛与铁皮石斛(D. catenatum)的分类问题, 学术界仍存在争议(Zhu et al, 2009; Xiang et al, 2013)。Zhu等(2009)基于假鳞茎、叶片和花朵等表型性状将霍山石斛和铁皮石斛合并在一起。后来, Xiang等(2013)基于4个叶绿体和ITS片段的系统学研究将霍山石斛与铁皮石斛划分为不同物种。就本质而言, 物种的表型性状是表型相关基因变异及表达调控的结果(Tanabe et al, 2005; Liao et al, 2019), 体现了遗传和环境因素的共同作用(Pigliucci et al, 2006; Leimar, 2009; 王姝和周道玮, 2017)。而分子系统学则是基于中性基因序列信息位点的变化揭示物种在进化时间尺度的先后次序(Zhu & Ge, 2005; Soto-Cerda et al, 2013; Xiang et al, 2016; Xiang et al, 2017)。因此, 基于分子系统学的结果并不能直接反映物种在表型性状方面的相似性, 需要对物种表型性状本身开展比较研究。
本研究选取霍山石斛的野生群体和随机人工授粉获得种子通过组织培养获得幼苗并种植在林间和温室环境下的F1代和F2代群体。同时选取相同人工栽培环境下与霍山石斛亲缘关系最近的河南石斛和表型相近的细茎石斛, 以及具有重要经济价值且与霍山石斛发生过混淆的铁皮石斛等物种, 对其营养器官和花部器官表型性状数据进行统计和分析。首次借鉴生态学同质园(common garden)实验(Montalvo & Ellstrand, 2000; Rutter & Fenster, 2007), 以及遗传学代际间表型性状比较的方法(Weber & Moorthy, 1952; Pande & Dhiman, 2011), 分别在群体水平对比分析环境因素和代际间遗传因素对霍山石斛群体表型性状影响的式样和规律, 以及相同环境下霍山石斛与近缘种群体表型性状之间的分化程度, 为霍山石斛及其近缘种的分类提供表型性状证据。
1 材料与方法
1.1 研究材料
本研究选取安徽霍山县太平畈乡16个石斛种植基地, 对野生霍山石斛(93株)、林间环境的F1代(203株)和F2代(221株)霍山石斛、温室环境的F1代(383株)和F2代(522株)霍山石斛、野生河南石斛(98株)、细茎石斛(280株)以及铁皮石斛(479株)共计2,279株植株进行采样, 对假鳞茎茎长和花瓣长等12个具有重要分类学意义的表型性状进行测量。由于开花的霍山石斛植株叶片多数脱落, 导致叶片表型信息缺失较多, 本文未加以分析。
野生霍山石斛于2017年采自安徽省霍山县的不同地点, 植株种植在安徽省霍山县长冲中药材开发有限公司的保种基地林间山坡上(约数百株)。此外, 保种基地内还有1980年以来采自太平畈乡三天门(约500 g)、五峰山(约250 g)和落儿岭(约1,850 g)等3个不同采集地的少量野生霍山石斛, 经随机人工授粉获得的种子进行组织培养产生F1代群体, 已在基地温室和林间环境下生长3年以上。该基地(占地面积约50亩)生境基本一致, 近似于生态学研究中的同质园条件。对F1代霍山石斛通过随机人工授粉获得的种子进行组织培养产生F2代群体, 分别种植在周边石斛种植基地温室和林间环境下2-3年。其中, F1代和F2代植株均不是无性繁殖, 并且没有经过人工选育, 可视为遗传学研究中的不同代际群体。
在石斛属系统发育关系的基础上结合物种表型性状的相似性, 选取了河南石斛和细茎石斛以及铁皮石斛等近缘种作为参考物种用于表型性状比较研究, 上述近缘种群体均在基地种植3年以上(附录1)。其中铁皮石斛种植在基地温室, 河南石斛和细茎石斛种植在林间环境下。由于基地种植规模较大, 本研究未对实际植株数量和种植面积进行详细统计。
1.2 数据统计分析
采取人工测量的方式获取上述样品表型性状数据, 使用Excel 2010对所有数据进行整理。
均值及差异显著性分析: 利用SPSS 19.0软件对表型性状数据进行均值统计, 使用Tamhane非参数法(Tamhane, 1981)对不同群体植株表型性状进行差异显著性检验。
利用SPSS 19.0软件对表型性状数据进行均值统计以及95%置信区间的计算, 用以检验各群体表型性状的间隔和重叠。
变异系数分析: 使用Excel 2010计算各表型性状变异系数, 用以评估各群体表型性状的变异程度。随后使用Excel 2010对各群体表型性状变异系数构建分布直方图并添加趋势线。其中, 变异系数计算公式为:
变异系数(CV) = 表型性状标准偏差/表型性状平均数
主成分分析: 利用SPSS 19.0软件对数据进行标准化的转换, 随后进行主成分分析, 选取前3个主成分因子, 累计贡献率不小于80%。基于成分得分矩阵获取各组分的表型性状的组成并计算各性状的累计贡献率。使用SPSS 19.0内置散点图以及图片编辑器分别对PC1和PC2、PC1和PC3作图并编辑。使用R语言ggplot2程序包对霍山石斛与近缘种群体营养器官和花部器官表型性状进行主成分分析作图, 并添加95%置信区间。
2 结果
2.1 不同环境对霍山石斛群体营养器官表型性状的影响
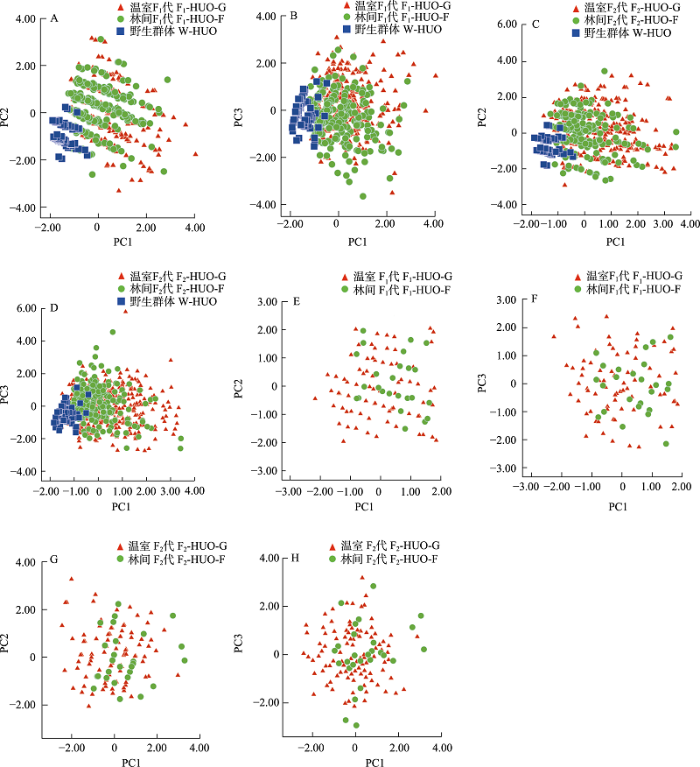
采用霍山石斛野生、林间F1代和温室F1代群体的营养器官表型性状数据进行种内表型差异分析(表1)。野生、林间F1代、温室F1代群体的平均假鳞茎茎长分别为1.84 cm、5.53 cm、5.80 cm。差异显著性及95%置信区间显示, 林间和温室F1代群体与野生群体之间存在极显著差异和间隔(表1, 附录2)。变异系数显示, 野生及林间和温室F1代群体霍山石斛表型性状变异程度近似(附录3)。对上述群体进行主成分分析, 选取前3个主成分因子, 累计贡献率为97.9%。其中, 野生群体植株整体聚集在一起, 林间和温室F1代群体混合在一起, 第一主成分包含假鳞茎茎长; 第二主成分包含假鳞茎直径; 第三主成分包含假鳞茎节长等性状(图1A, B)。
表1 野生及不同环境下F1代和F2代霍山石斛假鳞茎与花部器官表型性状差异
Table 1
| 野生型 Wild type | F1代 F1 generation | F2代 F2 generation | ||||
|---|---|---|---|---|---|---|
| 林间 Woodland | 温室 Greenhouse | 林间 Woodland | 温室 Greenhouse | |||
| 假鳞茎数量 Number of pseudobulbs | 93 | 202 | 355 | 221 | 500 | |
| 假鳞茎茎长 Pseudobulb length (cm) | 1.84 ± 0.85A | 5.53 ± 2.59BE | 5.80 ± 2.65BCE | 4.92 ± 2.28BD | 5.77 ± 2.83BCE | |
| 假鳞茎直径 Pseudobulb diameter (cm) | 0.45 ± 0.07A | 0.70 ± 0.12B | 0.69 ± 0.13B | 0.58 ± 0.13C | 0.59 ± 0.13C | |
| 假鳞茎茎长直径比 Length/diameter ratio of pseudobulb | 4.17 ± 2.03A | 7.98 ± 3.70B | 8.64 ± 4.24B | 9.13 ± 5.07BC | 10.16 ± 5.55C | |
| 假鳞茎节长 Internode length of pseudobulb (cm) | 0.65 ± 0.18A | 1.14 ± 0.32BE | 1.26 ± 0.36CE | 1.18 ± 0.30BcDE | 1.20 ± 0.39BCDE | |
| 假鳞茎节数 Number of pseudobulb internodes | 3.67 ± 0.81A | 6.02 ± 1.46BE | 5.75 ± 1.28Bce | 5.48 ± 1.40C | 6.05 ± 1.62BDE | |
| 花朵数量 Number of flowers | 2 | 26 | 107 | 31 | 151 | |
| 中萼片长 Dorsal sepal length (cm) | 1.35 ± 0.07A | 1.41 ± 0.16AB | 1.29 ± 0.19AC | 1.51 ± 0.26ABD | 1.44 ± 0.23ABD | |
| 中萼片宽 Dorsal sepal width (cm) | 0.60 ± 0AE | 0.68 ± 0.09Be | 0.66 ± 0.11BC | 0.66 ± 0.10aBCDE | 0.61 ± 0.09AbDE | |
| 中萼片长宽比 Length/width ratio of dorsal sepal | 2.25 ± 0.11A | 2.11 ± 0.34AB | 2.02 ± 0.34ABC | 2.29 ± 0.38ABD | 2.39 ± 0.44AD | |
| 花瓣长 Petal length (cm) | 1.40 ± 0A | 1.37 ± 0.15AB | 1.28 ± 0.21BC | 1.47 ± 0.24ABD | 1.42 ± 0.22ABD | |
| 花瓣宽 Petal width (cm) | 0.80 ± 0A | 0.80 ± 0.11A | 0.77 ± 0.14A | 0.76 ± 0.13AD | 0.68 ± 0.13d | |
| 花瓣长宽比 Length/width ratio of petal | 1.75 ± 0A | 1.74 ± 0.28AB | 1.71 ± 0.30ABC | 1.99 ± 0.41aBcD | 2.13 ± 0.39D | |
| 花梗长 Pedicel length (cm) | 2.15 ± 0.07A | 1.93 ± 0.46A | 2.11 ± 0.44A | 2.07 ± 0.43A | 2.17 ± 0.51A | |
同一行相同大写或小写字母为无显著性差异(P > 0.05), 不同大写字母为极显著性差异(P < 0.01), 不同小写字母为显著性差异(P < 0.05)。
The identical superscript capital or lowercase letter in the same row indicates no significant difference (P > 0.05), the different superscript capital letters in the same row indicate extremely significant difference (P < 0.01), the different superscript lowercase letters in the same row indicate significant difference (P < 0.05).
图1
图1
不同环境条件下霍山石斛群体假鳞茎和花部表型性状主成分分析。(A)-(B)林间和温室环境下, F1代假鳞茎表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图; (C)-(D)林间和温室环境下, F2代假鳞茎表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图; (E)-(F)林间和温室环境下, F1代花部表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图; (G)-(H)林间和温室环境下, F2代花部表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图。
Fig. 1
Principal component analysis of pseudobulbs and flowers of Dendrobium huoshanense in different environmental conditions. A-B, PCA of pseudobulbs in wild-type (W-HUO), F1 generation under woodland (F1-HUO-F) and greenhouse (F1-HUO-G); C-D, PCA of pseudobulbs in F2 generation under woodland (F2-HUO-F) and greenhouse (F2-HUO-G); E-F, PCA of flowers in F1 generation under woodland (F1-HUO-F) and greenhouse (F1-HUO-G); G-H, PCA of flowers in F2 generation under woodland (F2-HUO-F) and greenhouse (F2-HUO-G).
对于霍山石斛野生、林间和温室F2代群体的营养器官表型性状数据进行种内表型差异分析(表1)。温室F2代群体平均假鳞茎茎长(5.77 cm)、假鳞茎直径(0.59 cm)、假鳞茎节长(1.20 cm)和假鳞茎节数(6.05)等性状均大于野生群体和林间F2代群体。差异显著性及95%置信区间显示, 林间F2代群体与野生群体之间呈极显著性差异且存在置信区间间隔; 林间F2代和温室F2代群体植株仅在假鳞茎茎长和假鳞茎节数呈现极显著性差异和间隔(表1, 附录2)。变异系数显示, 温室F2代群体比林间F2代群体具有相对较大的变异程度(附录3)。在上述群体主成分分析中, 选取前3个主成分因子, 累计贡献率为97.5%。林间和温室F2代群体均存在较大的变化范围以及重叠的比例。第一主成分包含假鳞茎茎长; 第二主成分包含假鳞茎直径; 第三主成分包含假鳞茎节长等表型性状(图1C, D)。
2.2 不同环境对霍山石斛群体花部器官表型性状的影响
采用林间和温室F1代群体的花部器官表型性状数据进行种内表型差异分析(表1)。林间和温室F1代群体的平均花瓣长分别为1.37 cm和1.28 cm。差异显著性和95%置信区间显示, 林间和温室F1代群体在中萼片长和宽以及花瓣长方面存在极显著性差异, 其中在中萼片长存在间隔(表1, 附录2)。变异系数结果显示, 温室F1代群体各性状变异系数均大于林间群体(附录3)。对上述群体进行主成分分析, 选取前3个主成分因子, 累计贡献率为96.5%。其中第一主成分为中萼片长和花瓣长; 第二主成分为中萼片宽和花瓣宽; 第三主成分为中萼片长宽比和花瓣长宽比。林间F1代和温室F1代群体花部表型性状均有较大的变化范围和重叠比例, 林间群体花瓣长和萼片长等性状呈现变小的趋势(图1E, F)。
采用林间和温室F2代群体花部器官表型性状数据进行种内表型差异分析(表1)。林间和温室F2代群体植株平均花瓣长分别为1.47 cm和1.42 cm。差异显著性和95%置信区间显示,林间和温室F2代群体在中萼片宽和花瓣宽等性状存在显著性差异, 95%置信区间在花瓣宽存在间隔(表1, 附录2)。变异系数显示,温室和林间F2代群体表型性状的变异程度近似(附录3)。对上述群体进行主成分分析, 选取前3个主成分因子, 累计贡献率为97.5%。其中, 第一主成分包含中萼片宽和花瓣宽; 第二主成分包含中萼片长宽比和花瓣长宽比; 第三主成分包含中萼片长和花瓣长。这两群体均具有较大的变化范围和重叠比例(图1G, H)。
2.3 代际间遗传因素对霍山石斛营养器官表型性状的影响
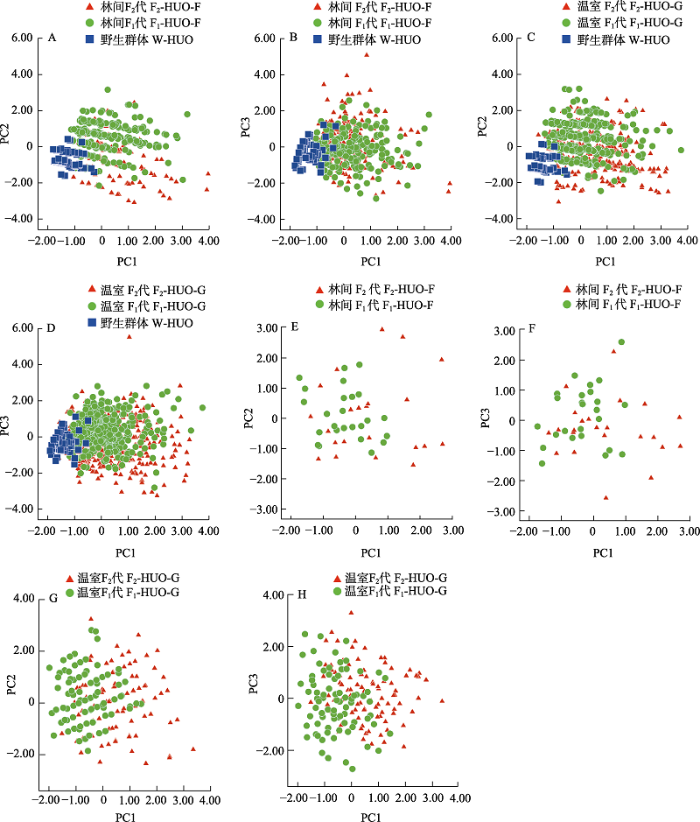
图2
图2
不同代际条件下霍山石斛群体假鳞茎和花部表型性状主成分分析。(A)‒(B)林间环境下, F1代和F2代假鳞茎表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图; (C)‒(D)温室环境下, F1代和F2代假鳞茎表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图; (E)‒(F)林间环境下, F1代和F2代花部表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图; (G)‒(H)温室环境下, F1代和F2代花部表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图。
Fig. 2
Principal component analysis of pseudobulbs and flowers of Dendrobium huoshanense in different generations. A‒B, PCA of pseudobulbs inwild-type (W-HUO), F1 (F1-HUO-F) and F2 generations (F2-HUO-F) under woodland; C‒D, PCA of pseudobulbs in D. huoshanense in wild-type (W-HUO), F1 (F1-HUO-G) and F2 generations (F2-HUO-G) under greenhouse; E‒F, PCA of flowers in F1 (F1-HUO-F) and F2 generations (F2-HUO-F) under woodland; G‒H, PCA of flowers in F1 (F1-HUO-G) and F2 generations (F2-HUO-G) under greenhouse.
温室环境下, F1代和F2代群体平均假鳞茎茎长分别为5.80 cm和5.77 cm, F2代群体整体假鳞茎节长较短、假鳞茎节数较多。差异显著性和95%置信区间显示, 除假鳞茎茎长和假鳞茎节长外, 温室F1代和F2代群体在其他性状上均呈极显著性差异, 其中在假鳞茎直径、假鳞茎茎长直径比以及假鳞茎节数方面存在间隔(表1, 附录2)。变异系数显示, 温室F2代相对F1代群体具有较大程度的变异(附录3)。对上述群体进行主成分分析, 选取前3个主成分因子, 累计贡献率为97.1%。第一主成分包含假鳞茎茎长; 第二主成分包含假鳞茎直径; 第三主成分包含假鳞茎节长和假鳞茎节数等性状。温室F1代与F2代群体均具有较大的变异范围和重叠比例(图2C, D)。
2.4 代际间遗传因素对霍山石斛花部器官表型性状的影响
林间环境下, F1代中萼片长和花瓣长分别为1.41 cm和1.37 cm, F2代群体中萼片长和花瓣长分别为1.51 cm和1.47 cm, F2代群体花朵相对较大。差异显著性和95%置信区间显示, 林间F1代和F2代群体在中萼片宽和花瓣长宽比性状方面存在显著性差异但不存在间隔(表1, 附录2)。变异系数显示,林间F2代群体相比F1代群体具有较大的变异程度(附录3)。对两群体主成分分析, 选取前3个主成分因子, 累计贡献率为97.6%。第一主成分为中萼片长和花瓣长; 第二主成分为中萼片宽和花瓣宽; 第三主成分为萼片长宽比和花瓣长宽比。林间F1代与F2代群体在第一主成分方面均有较大的变异范围, 两群体有着较大比例重叠(图2E, F)。
温室条件下, F1代群体中萼片长和花瓣长分别为1.29 cm和1.28 cm, F2代中萼片长和花瓣长分别为1.44 cm和1.42 cm, F2代花朵相对较大。差异显著性和95%置信区间显示, 除花梗长外, 温室F1代与F2代群体花部表型性状均呈极显著性差异和间隔(表1, 附录2)。变异系数显示,温室F1代和F2代群体的花部表型性状则有着相近的变异程度(附录3)。对两群体花部表型性状主成分分析, 选取前3个主成分因子, 累计贡献率为97.0%。第一主成分为中萼片长宽比和花瓣长宽比; 第二主成分为花瓣宽和中萼片宽等; 第三主成分为花瓣长和中萼片长等性状。温室F1代与F2代在第一主成分方面均有较大的变异范围, 两群体之间存在一定程度重叠(图2G, H)。
2.5 霍山石斛及其近缘种群体营养器官表型性状分化
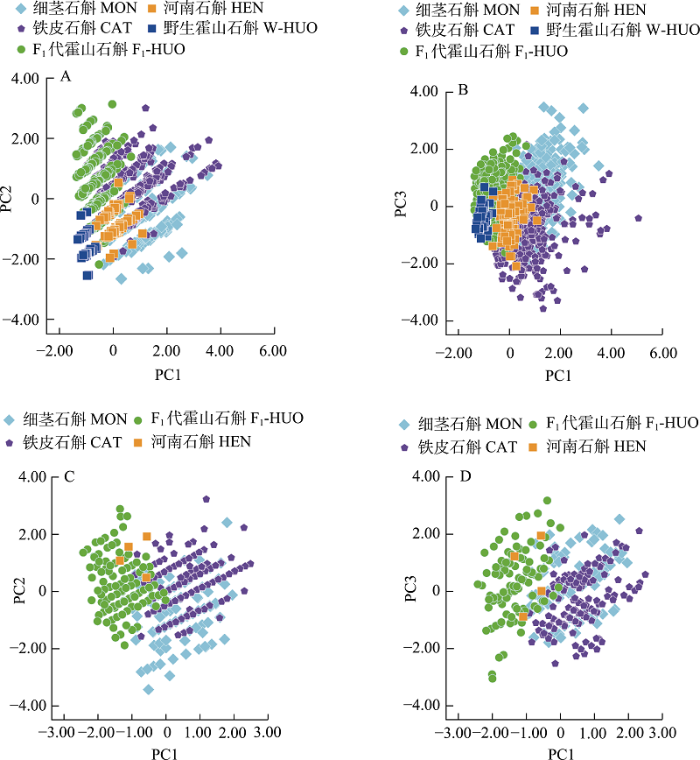
利用霍山石斛(野生群体、F1代和F2代所有群体)、河南石斛、细茎石斛以及铁皮石斛群体的营养器官表型性状数据进行种间表型差异分析(表2)。细茎石斛平均假鳞茎茎长最长(18.54 cm), 野生霍山石斛群体平均假鳞茎茎长最短(1.84 cm); 河南石斛、铁皮石斛平均假鳞茎茎长居中, 分别为8.76 cm和14.97 cm。差异显著性及95%置信区间显示, 野生霍山石斛与F1代和F2代霍山石斛群体均呈极显著性差异和间隔, F1代和F2代霍山石斛群体仅在假鳞茎直径和假鳞茎茎长直径比方面存在极显著性差异和间隔。野生霍山石斛与河南石斛、细茎石斛以及铁皮石斛之间整体均呈极显著性差异和间隔。F1代和F2代霍山石斛与河南石斛等近缘种之间均呈极显著性差异和间隔。河南石斛与细茎石斛以及铁皮石斛两两之间整体均呈显著或极显著性差异和间隔(表2, 附录4)。变异系数显示, 铁皮石斛群体植株假鳞茎表型性状整体变异程度最大, 野生霍山石斛及其子代群体变异程度其次, 河南石斛表型性状变异程度最小(附录5)。对上述近缘种群体进行主成分分析, 选取前3个主成分因子, 累计贡献率为97.8%。第一主成分包含假鳞茎茎长; 第二主成分包含假鳞茎直径; 第三主成分包含假鳞茎节长和假鳞茎节数等性状。各群体整体各自聚集在一起, 相互之间有着不同程度的重叠。野生霍山石斛群体单独聚在一起且变化范围较小, 其他近缘种群体则具有广泛的变化范围。在第一和第三主成分方面野生霍山石斛、细茎石斛、铁皮石斛等之间呈现较为明显的分化(图3A, B, 附录6)。
表2 霍山石斛及其近缘种假鳞茎与花部表型性状差异
Table 2
| 霍山石斛 D. huoshanense | 河南石斛 D. henanense | 细茎石斛 D. moniliforme | 铁皮石斛 D. catenatum | |||
|---|---|---|---|---|---|---|
| Wild type | F1 generation | F2 generation | ||||
| 假鳞茎数量 No. of pseudobulbs | 93 | 557 | 721 | 98 | 217 | 414 |
| 假鳞茎茎长 Pseudobulb length (cm) | 1.84 ± 0.85A | 5.70 ± 2.63B | 5.51 ± 2.70B | 8.76 ± 2.12C | 18.54 ± 5.87E | 14.97 ± 8.26D |
| 假鳞茎直径 Pseudobulb diameter (cm) | 0.45 ± 0.07A | 0.70 ± 0.13B | 0.59 ± 0.13C | 0.43 ± 0.06AD | 0.39 ± 0.09F | 0.53 ± 0.11E |
| 假鳞茎茎长直径比 Length/diameter ratio of pseudobulb | 4.17 ± 2.03A | 8.40 ± 4.06B | 9.84 ± 5.42C | 20.75 ± 5.90D | 48.99 ± 16.88F | 30.16 ± 19.40E |
| 假鳞茎节长 Internode length of pseudobulb (cm) | 0.65 ± 0.18A | 1.21 ± 0.35B | 1.19 ± 0.36B | 1.52 ± 0.32C | 2.38 ± 0.55D | 1.65 ± 0.57c |
| 假鳞茎节数 No. of pseudobulb internodes | 3.67 ± 0.81A | 5.85 ± 1.35B | 5.87 ± 1.58B | 8.45 ± 1.44C | 10.42 ± 2.30d | 11.17 ± 3.21D |
| 花朵数量 Number of flowers | 2 | 132 | 181 | 5 | 138 | 235 |
| 中萼片长 Dorsal sepal length (cm) | 1.35 ± 0.07A | 1.32 ± 0.19AB | 1.45 ± 0.23AC | 1.62 ± 0.15ABCD | 1.47 ± 0.27ACD | 1.77 ± 0.23ADE |
| 中萼片宽 Dorsal sepal width (cm) | 0.60 ± 0A | 0.66 ± 0.11B | 0.62 ± 0.09abC | 0.70 ± 0.09ABCD | 0.51 ± 0.08DF | 0.63 ± 0.07bCDE |
| 中萼片长宽比 Length/width ratio of dorsal sepal | 2.25 ± 0.11A | 2.03 ± 0.34AB | 2.37 ± 0.43AC | 2.35 ± 0.31ABCD | 2.90 ± 0.45ADE | 2.83 ± 0.40ADE |
| 花瓣长 Petal length (cm) | 1.40 ± 0A | 1.30 ± 0.20B | 1.43 ± 0.22ACF | 1.52 ± 0.07AbCDF | 1.49 ± 0.24DF | 1.67 ± 0.20DE |
| 花瓣宽 Petal width (cm) | 0.80 ± 0A | 0.77 ± 0.14AB | 0.69 ± 0.13C | 0.82 ± 0.04ABcD | 0.52 ± 0.09F | 0.56 ± 0.07E |
| 花瓣长宽比 Length/width ratio of petal | 1.75 ± 0A | 1.72 ± 0.29AB | 2.11 ± 0.40C | 1.86 ± 0.16ABCD | 2.92 ± 0.47E | 3.01 ± 0.46E |
同一行相同大写或小写字母为无显著性差异(P > 0.05), 不同大写字母为极显著性差异(P < 0.01), 不同小写字母为显著性差异(P < 0.05)。
The identical superscript capital or lowercase letter in the same row indicates no significant difference (P > 0.05), the different superscript capital letters in the same row indicate extremely significant difference (P < 0.01), the different superscript lowercase letters in the same row indicate significant difference (P < 0.05).
图3
图3
野生霍山石斛(W-HUO)、F1代霍山石斛(F1-HUO)、河南石斛(HEN)、细茎石斛(MON)以及铁皮石斛(CAT)群体假鳞茎和花部表型性状主成分分析。(A)-(B)霍山石斛及其近缘种群体假鳞茎表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图; (C)-(D)霍山石斛及其近缘种群体花部表型性状主成分(PC1 vs. PC2)和(PC1 vs. PC3)散点图。
Fig. 3
Principal component analysis (PCA) of phenotypic traits of pseudobulbs and flowers in Dendrobium huoshanense in wild-type (W-HUO), F1 generation of D. huoshanense (F1-HUO), D. henanense (HEN), D. moniliforme (MON) and D. catenatum (CAT). A‒B, PCA of pseudobulbs in D. huoshanense and related species; C‒D, PCA of flowers in D. huoshanense and related species.
2.6 霍山石斛及其近缘种群体花部器官表型性状分化
利用霍山石斛(F1代和F2代所有群体)、河南石斛、细茎石斛以及铁皮石斛群体的花部器官表型性状数据进行种间表型差异分析(表2)。结果显示, 铁皮石斛花瓣长最大, 为1.67 cm; F1代霍山石斛花瓣长最短, 为1.30 cm; 河南石斛与细茎石斛群体花瓣长度居中, 分别为1.52 cm和1.49 cm。差异显著性和95%置信区间显示, F1代和F2代霍山石斛之间, 除花梗长外, 其余表型性状均呈显著性或极显著性差异和间隔。F1代霍山石斛与铁皮石斛相比, 除中萼片宽呈显著性差异外, 其余性状均呈极显著性差异和间隔。F1代霍山石斛与细茎石斛相比, 除花梗长外, 其余性状均呈极显著性差异和间隔。F1代霍山石斛与河南石斛相比, 仅在花瓣长存在显著性差异和间隔(表2, 附录4)。变异系数显示, F1代和F2代霍山石斛以及细茎石斛群体整体花部表型性状变异程度最大, 河南石斛变异程度最小(附录5)。对上述物种进行主成分分析, 选取前3个主成分因子, 累计贡献率为97.6%。第一主成分包含中萼片长宽比和花瓣长宽比; 第二主成分包含中萼片宽和花瓣宽; 第三主成分包含中萼片长和花瓣长。F1代霍山石斛、河南石斛、铁皮石斛以及细茎石斛群体整体各自聚在一起且具有相对较大的变化范围, 群体之间存在不同程度的重叠。霍山石斛与河南石斛等群体在第一和第二主成分方面呈现较为明显的分化(图3C, D, 附录7)。
3 讨论
3.1 环境差异对野生、林间子代、温室子代霍山石斛群体营养器官表型分化有显著影响
表型是植物适应环境的结果(Sultan, 2000; Siefert et al, 2014; Fox et al, 2019)。在不同的环境中, 植物经历不同选择压力, 进而产生相应的表型变异以适应新环境(李磊等, 2010; 刘龙昌等, 2015), 这对于揭示物种表型变化以及物种环境适应性等物种问题的研究具有重要意义(Valladares et al, 2006; Gienapp et al, 2008; Anderson et al, 2012)。我们的研究发现环境差异对霍山石斛表型性状存在较大影响。野生环境下霍山石斛群体植株矮小, 而人工环境下(林间和温室)的F1代和F2代整体呈现假鳞茎变高以及假鳞茎直径变大等趋势。对于林间和温室F1代群体以及林间和温室F2代群体而言, 霍山石斛在假鳞茎茎长等性状方面产生了显著性差异。
一般地, 植物营养器官比花部器官更易受环境的影响(邢毅等, 2008), 环境差异导致的选择压力促使营养器官和花部器官表型性状产生不同程度的变异, 从而有助于提高其适合度(Schlichting & Levin, 1984)。与花部器官相比, 营养器官表型性状在野生或子代霍山石斛群体中具有较高的变异程度以及较好的种内区分效率, 这或许与营养器官表型更易受选择压力影响有关。在非生物因素方面, 野生霍山石斛生长于强光照、温差大和缺乏营养的崖壁上, 环境选择压力较大, 植株整体较为矮小。在人工环境中, 由于营养、水分等充足且温度适宜, 植株表型性状会产生假鳞茎茎长变长、假鳞茎直径变粗的趋势, 同时具有较高的表型多样性。在生物因素方面, 共生真菌通过影响石斛种子的萌发间接影响群体表型多样性。共生真菌对于兰科植物的生长和发育是必需的(Rasmussen, 1995), 这种共生关系涉及营养物质的交换(Smith & Read, 2008)。由于石斛种子数量多且无胚乳, 野外植株种子萌发率极低, 仅少数与真菌共生萌发后形成的植株可以存活, 这极大地限制了野生群体规模以及群体植株的表型多样性。在人工干预条件下, 种子萌发率接近100%, 进而促进了子代群体规模的扩大以及表型多样性的提高。所以, 我们认为生物因素和非生物因素的共同作用可能是促使霍山石斛植株营养器官表型在人工和野生环境下产生差异的重要原因。
3.2 遗传因素在F1代和F2代霍山石斛群体表型分化方面具有一定程度的影响
遗传因素对表型同样有着重要的影响(Pintado et al, 1997; Mitchell-Olds et al, 2007; García-Verdugo et al, 2009, 2010)。一般地, 相同环境下, 代际间的差异通常反映出遗传层面的变化。在自然界中, 由于植物的特殊性, 同一群体内代际共存是一种普遍现象(Fan et al, 1992)。本研究中, 无论在温室还是林间环境, F2代相比F1代霍山石斛群体均呈现假鳞茎直径变细且假鳞茎茎长直径比增大的趋势和显著性差异。已有研究显示, 茎秆(假鳞茎)伸长以及茎秆(假鳞茎)居间分生组织生长受到多种内源激素调控, 包括赤霉素(van der Knaap & Kende, 2000; Bashline et al, 2014; Huang et al, 2015; Marowa et al, 2016)、油菜素内酯(Wang et al, 2012; Guo et al, 2013)和生长素(Schenck et al, 2010)。因而我们推测这种代际群体间呈现的表型性状差异或许与调控营养器官表型相关基因在群体水平的变异有关。
造成上述现象的原因, 客观方面或许与调控营养器官表型相关基因的非同义突变或基因型频率在群体水平的变化有关。主观方面人工授粉果实数量对不同代际群体基因型频率的变化有较大的影响。F2代群体主要来源于在F1代群体的随机人工授粉获得的果实, 但通常组织培养所需的果实数量有限, 这在一定程度上容易造成F2代获得F1代群体部分种类的基因型或获得的基因型频率发生偏差。
3.3 霍山石斛在表型层面是区别于其他近缘种的独立物种
霍山石斛与近缘种群体之间存在显著的表型分化。在营养器官方面, 霍山石斛与近缘种群体在所有表型性状方面均呈显著分化。在花部器官方面, 霍山石斛群体与细茎石斛和铁皮石斛群体花瓣长和宽等多数性状呈现显著分化, 但与河南石斛群体整体分化不显著。因此, 营养器官和花部器官表型性状分析结果均支持霍山石斛作为区别于细茎石斛和铁皮石斛的独立物种。但是, 仅营养器官的表型性状结果支持霍山石斛作为区别于河南石斛的独立物种。
营养器官的表型性状相对花部器官具有更高的种间区分效率。霍山石斛与铁皮石斛等群体在营养器官表型性状方面均呈显著分化且具有相对较高的变异程度, 而花部器官部分表型性状分化不彻底且整体变异程度相对较低, 这或许与营养器官和花部器官面临不同的选择压力有关。一般地, 花部器官更多地受传粉者选择的影响(Herrera et al, 2006; Harder & Johnson, 2009; Sletvold et al, 2010)。赤霉素、油菜素内酯以及生长素等内源激素相关基因可以调控植株茎秆(假鳞茎)居间分生组织以及茎秆(假鳞茎)伸长等(Wang et al, 2018), 而相对保守的MADS-BOX等基因家族部分成员则参与调控花部器官的表型和发育(Pelaz et al, 2000; Kater et al, 2006)。这些不同的基因通常具有不同的进化历史和进化速率(Zhu & Ge, 2005; Soto-Cerda et al, 2013), 进而很大程度上影响着植物营养器官和花部器官表型的分化程度。
较短的进化历史和有限的样品数量是石斛属物种边界存在重叠和争议的客观及主观原因。已有研究显示, 霍山石斛与其他近缘种的分化主要集中在更新世, 而霍山石斛与河南石斛之间分化时间更晚, 约在更新世末期(徐晴, 2015; Xiang et al, 2016)。较短的分化时间往往伴随着物种表型及遗传层面分化不彻底, 从而很大程度上影响表型性状的间隔和分化。因此, 霍山石斛与河南石斛花部器官表型性状方面未产生显著分化的客观原因可能与物种较短的分化时间有关。此外, 本研究中, 野生霍山石斛与河南石斛群体花朵数量分别为2朵和5朵, 较少的样品数量直接导致花部器官表型性状95%置信区间的扩大, 进而容易造成对物种的比较和归类产生偏差。因此, 对于野生霍山石斛和河南石斛群体花部器官表型分化问题, 仍需要进一步扩大花朵样本量。
3.4 群体表型性状研究重点关注分类学中的比较和归类方面
霍山石斛与河南石斛和铁皮石斛等近缘种分类问题存在广泛的争议, 需要对物种表型性状本身进行深入研究。在已有的研究中, Zhu等(2009)基于营养器官和花部器官等表型性状将霍山石斛与铁皮石斛合并为同一物种, 而分子系统学则将霍山石斛与铁皮石斛分为不同物种(Zhu et al, 2009; Xiang et al, 2013), 这种分歧与植物表型性状的变化程度存在密切联系。在自然界中, 植物个体表型性状通常受遗传和环境因素的影响(Pigliucci et al, 2006; Leimar, 2009; 王姝和周道玮, 2017), 进而产生表型可塑性以及代际间遗传因素导致的表型变化, 这是植物长期适应环境的结果(Pigliucci et al, 2006; Fox et al, 2019)。这种表型性状的变化对分类学研究中的比较和归类问题产生了很大困扰。由于单个个体水平表型性状的描述无法反映出环境和遗传因素对表型性状的影响, 所以对于争议物种界定问题, 需要从群体水平开展物种表型性状的比较分析。
群体表型性状的研究可以为分类学中比较和归类方面的争议提供解决方案。对于霍山石斛与河南石斛和铁皮石斛等近缘种的分类学争议问题, 厘清环境因素和遗传因素对物种表型性状影响的程度和规律是关键。我们在前人分类学研究的基础上, 从群体水平对不同环境以及代际间物种表型性状进行比较研究, 重点探讨物种表型性状的比较和归类等方面。首次单独解析了环境因素对表型性状的影响式样以及代际间遗传因素对表型性状的影响模式, 这为物种的比较和归类研究提供了可靠的证据。本研究通过对霍山石斛开展群体水平同质园实验和代际间对比以及与河南石斛和铁皮石斛等近缘种表型性状比较, 明确了环境因素和代际间遗传因素对霍山石斛表型性状的影响程度和规律以及霍山石斛与近缘种之间的分化状态, 确定了霍山石斛表型性状的变异幅度及变异规律, 解决了霍山石斛与河南石斛和铁皮石斛等近缘种的比较和归类问题。
我们的研究结果表明, 对于分类学研究中的争议问题, 需要对物种的表型性状进行深入比较和分析, 在明确环境和遗传因素对群体表型性状影响程度的基础上, 最终提出较理想的物种鉴定和识别问题解决方案。未来还需要采用更多分子片段甚至在系统发育基因组层面解析霍山石斛等近缘物种的系统发育关系, 结合表型变化规律, 探讨这些类群的物种形成等进化问题。在此, 我们呼吁在未来的分类学研究领域重点关注物种表型性状的变化式样, 从而为分类学发展提供更好的基础。
附录 Supplementary Material
附录1 种植基地内霍山石斛及其近缘种植株形态照片
Appendix 1 Photos of Dendrobium huoshanense and related species in dendrobiums planting base
附录2 霍山石斛群体假鳞茎和花部表型性状95%置信区间
Appendix 2 The 95% confidence interval for phenotypic traits of pseudobulbs and flowers in Dendrobium huoshanense
附录3 霍山石斛群体假鳞茎和花部表型性状变异系数
Appendix 3 Phenotypic variation coefficients of pseudobulbs and flowers traits in Dendrobium huoshanense
附录4 霍山石斛及其近缘种群体假鳞茎和花部表型性状95%置信区间
Appendix 4 The 95% confidence interval for phenotypic traits of pseudobulbs and flowers in Dendrobium huoshanense and related species
附录5 霍山石斛及其近缘种群体假鳞茎和花部表型性状变异系数
Appendix 5 Phenotypic variation coefficients of pseudobulbs and flowers traits in Dendrobium huoshanense and related species
附录6 野生霍山石斛、F1代霍山石斛、河南石斛、细茎石斛以及铁皮石斛群体假鳞茎表型性状主成分分析散点图
Appendix 6 Scatter plots of the principal component analysis of pseudobulbs in Dendrobium huoshanense in wild-type, F1 generation of D. huoshanense, D. henanense, D. moniliforme and D. catenatum
附录7 F1代霍山石斛、河南石斛、细茎石斛以及铁皮石斛群体花部表型性状主成分分析散点图
Appendix 7 Scatter plots of the principal component analysis of flowers in F1 generation of Dendrobium huoshanense, D. henanense, D. moniliforme and D. catenatum
致谢
感谢安徽省霍山县长冲中药材开发有限公司何祥林提供宝贵的野生霍山石斛群体材料; 感谢安徽省霍山县太平畈乡康顺公司等16家石斛种植企业或基地提供大量的霍山石斛及近缘种群体材料保障本研究的顺利完成; 感谢鲁宾雁博士、张武凡博士、胡超博士和邹玉方师妹等课题组成员在稿件修改中的帮助; 感谢任之尧博士(暨南大学)在稿件修改中的帮助并提出了宝贵的建议。
参考文献
Systematics of Dendrobiinae (Orchidaceae), with special reference to Australian taxa
DOI:10.1111/boj.2011.166.issue-2 URL [本文引用: 1]
Cell wall, cytoskeleton, and cell expansion in higher plants
DOI:10.1093/mp/ssu018
PMID:24557922
[本文引用: 1]

To accommodate two seemingly contradictory biological roles in plant physiology, providing both the rigid structural support of plant cells and the adjustable elasticity needed for cell expansion, the composition of the plant cell wall has evolved to become an intricate network of cellulosic, hemicellulosic, and pectic polysaccharides and protein. Due to its complexity, many aspects of the cell wall influence plant cell expansion, and many new and insightful observations and technologies are forthcoming. The biosynthesis of cell wall polymers and the roles of the variety of proteins involved in polysaccharide synthesis continue to be characterized. The interactions within the cell wall polymer network and the modification of these interactions provide insight into how the plant cell wall provides its dual function. The complex cell wall architecture is controlled and organized in part by the dynamic intracellular cytoskeleton and by diverse trafficking pathways of the cell wall polymers and cell wall-related machinery. Meanwhile, the cell wall is continually influenced by hormonal and integrity sensing stimuli that are perceived by the cell. These many processes cooperate to construct, maintain, and manipulate the intricate plant cell wall--an essential structure for the sustaining of the plant stature, growth, and life.
Just how many orchids are there? In: Proceedings of the 18th World Orchid Conference (eds Raynal-Roques A, Roguenant A, Prat D)
A study on the species group age structure ofLarix gmelini population and its relation to disturbance in the north Daxinganling Mountains
大兴安岭北部兴安落叶松种群年龄结构及其与自然干扰关系的研究
The development of classical plant taxonomy and iFlora
DOI:10.3724/SP.J.1143.2012.12144 URL [本文引用: 1]
经典植物分类学的发展与iFlora
Beyond buying time: The role of plasticity in phenotypic adaptation to rapid environmental change
DOI:10.1098/rstb.2018.0174 URL [本文引用: 2]
Genetic diversity and differentiation processes in the ploidy series ofOlea europaea L.: A multiscale approach from subspecies to insular populations
DOI:10.1111/j.1365-294X.2008.04027.x
PMID:19143937
[本文引用: 1]

Geographical isolation and polyploidization are central concepts in plant evolution. The hierarchical organization of archipelagos in this study provides a framework for testing the evolutionary consequences for polyploid taxa and populations occurring in isolation. Using amplified fragment length polymorphism and simple sequence repeat markers, we determined the genetic diversity and differentiation patterns at three levels of geographical isolation in Olea europaea: mainland-archipelagos, islands within an archipelago, and populations within an island. At the subspecies scale, the hexaploid ssp. maroccana (southwest Morocco) exhibited higher genetic diversity than the insular counterparts. In contrast, the tetraploid ssp. cerasiformis (Madeira) displayed values similar to those obtained for the diploid ssp. guanchica (Canary Islands). Geographical isolation was associated with a high genetic differentiation at this scale. In the Canarian archipelago, the stepping-stone model of differentiation suggested in a previous study was partially supported. Within the western lineage, an east-to-west differentiation pattern was confirmed. Conversely, the easternmost populations were more related to the mainland ssp. europaea than to the western guanchica lineage. Genetic diversity across the Canarian archipelago was significantly correlated with the date of the last volcanic activity in the area/island where each population occurs. At the island scale, this pattern was not confirmed in older islands (Tenerife and Madeira), where populations were genetically homogeneous. In contrast, founder effects resulted in low genetic diversity and marked genetic differentiation among populations of the youngest island, La Palma.
Contrasting patterns of morphological and physiological differentiation across insular environments: Phenotypic variation and heritability of light-related traits inOlea europaea
DOI:10.1007/s00442-010-1672-7
PMID:20532918
[本文引用: 1]

Phenotypic variation of traits can reflect the ability of plants to adjust to particular environments, but how much of this variation is heritable is not frequently analyzed in natural populations. In the present paper, we investigated the patterns of phenotypic expression in light-related leaf traits of Olea europaea subsp. guanchica, a woody sclerophyllous species endemic to the Canary Islands. We explored phenotypic differentiation and heritable variation across several island populations differing in light environment. A suite of morpho-functional (leaf size, SLA and leaf angle) and physiological (pigment pools: Chl a/b ratio, xantophyll cycle and β-carotene) traits was measured in six populations on three islands. In addition, we estimated heritabilities for these traits following Ritland's method. Variation in morpho-functional, but not in physiological, traits was observed across the islands and was significantly related to the amount of diffuse light experienced by each population. In addition, significant heritabilities were found for morpho-functional traits, whereas expression of similar phenotypes among populations was accompanied by a lack of heritable variation in physiological traits. Most recently established populations did not exhibit lower heritabilities in quantitative traits than older populations, and apparently displayed congruent phenotypes under the local conditions. Our results strongly support the idea that different types of traits show contrasted levels of genetic and phenotypic variation in populations experiencing marked environmental differences.
What determines species diversity?
什么决定了物种的多样性?
Climate change and evolution: Disentangling environmental and genetic responses
DOI:10.1111/j.1365-294X.2007.03413.x
PMID:18173499
[本文引用: 1]

Rapid climate change is likely to impose strong selection pressures on traits important for fitness, and therefore, microevolution in response to climate-mediated selection is potentially an important mechanism mitigating negative consequences of climate change. We reviewed the empirical evidence for recent microevolutionary responses to climate change in longitudinal studies emphasizing the following three perspectives emerging from the published data. First, although signatures of climate change are clearly visible in many ecological processes, similar examples of microevolutionary responses in literature are in fact very rare. Second, the quality of evidence for microevolutionary responses to climate change is far from satisfactory as the documented responses are often - if not typically - based on nongenetic data. We reinforce the view that it is as important to make the distinction between genetic (evolutionary) and phenotypic (includes a nongenetic, plastic component) responses clear, as it is to understand the relative roles of plasticity and genetics in adaptation to climate change. Third, in order to illustrate the difficulties and their potential ubiquity in detection of microevolution in response to natural selection, we reviewed the quantitative genetic studies on microevolutionary responses to natural selection in the context of long-term studies of vertebrates. The available evidence points to the overall conclusion that many responses perceived as adaptations to changing environmental conditions could be environmentally induced plastic responses rather than microevolutionary adaptations. Hence, clear-cut evidence indicating a significant role for evolutionary adaptation to ongoing climate warming is conspicuously scarce.
Mechanisms and networks for brassinosteroid regulated gene expression
DOI:10.1016/j.pbi.2013.08.002 URL [本文引用: 1]
Darwin's beautiful contrivances: Evolutionary and functional evidence for floral adaptation
DOI:10.1111/nph.2009.183.issue-3 URL [本文引用: 1]
Geographical context of floral evolution:Towards an improved research programme in floral diversification
Biodiversity pursuits need a scientific and operative species concept
DOI:10.17520/biods.2016203 URL [本文引用: 1]
生物多样性事业需要科学、可操作的物种概念
DOI:10.17520/biods.2016203
[本文引用: 1]

物种概念(species concept)是生物学家们持续关注的中心问题。物种概念决定物种划分, 而物种划分的合理性关系到生物多样性的研究、保护和可持续利用。本文把现有较流行的物种概念分为6类, 并对它们予以述评后指出: 虽然生物学物种概念、遗传学物种概念、进化物种概念、系统发生物种概念等从不同方面认识了物种的客观真实性和物种的本质, 但在实践中都难以操作。绝大多数物种是由分类学家划分的, 但目前所有的分类学物种概念都包含有不同程度的主观因素, 从而造成物种划分的人为性, 对生物多样性研究造成负面影响。因此, 生物多样性事业需要科学、可操作的物种概念。本文在吸收了生物学物种概念、遗传学物种概念、进化物种概念以及系统发生物种概念等的长处, 也分析了它们的不足和问题的基础上提出一个新的物种概念, 即形态-生物学物种概念。最后, 以芍药属(Paeonia)几个物种的处理为例, 说明这一新的物种概念是可操作的, 划分的物种在形态上区别分明, 易于鉴别。更重要的是, 其结果得到基于25或26个单拷贝或寡拷贝核基因DNA序列所作的系统发生分析的强有力支持。各个物种在系统发生树上形成单系和独立的谱系, 表明其间各自形成独立的基因库, 没有基因交换, 它们独立进化, 有各自的生态位和独立的分布区。因此, 利用这一新的物种概念能够达到预期目标。
A gibberellin-mediated DELLA-NAC signaling cascade regulates cellulose synthesis in rice
Taxonomic revision ofDendrobium moniliforme complex (Orchidaceae)
DOI:10.1016/j.scienta.2008.10.002 URL [本文引用: 2]
Functional conservation of MADS-box factors controlling floral organ identity in rice andArabidopsis
DOI:10.1093/jxb/erl097 URL [本文引用: 1]
Biodiversity undertakings call for extensive discussion on species concept and the criteria for species delimitation
DOI:10.17520/biods.2016291 URL [本文引用: 1]
生物多样性事业呼唤对物种概念和物种划分标准的深度讨论
Environmental and genetic cues in the evolution of phenotypic polymorphism
DOI:10.1007/s10682-007-9194-4 URL [本文引用: 3]
Phenotypic variation and covariation among natural populations ofArabidopsis thaliana in North Xinjiang
DOI:10.3724/SP.J.1003.2010.497 URL [本文引用: 1]
新疆北部拟南芥自然居群表型变异与协变
DOI:10.3724/SP.J.1003.2010.497
[本文引用: 1]

拟南芥(Arabidopsis thaliana)自然居群的表型特征代表其在自然环境下的适应状况, 不同居群间特征的对比可以为了解拟南芥表型变化规律, 进而分析其形成过程和机制提供重要线索。本研究以分布于新疆北部天山、塔尔巴哈台山和阿尔泰山的10个种群的9个表型性状为基础, 对比分析了小尺度、局域尺度和区域尺度环境下原生境拟南芥种群表型性状的变化。结果发现, 不同性状对环境变化的反应不同, 其中株高、株重、根重、根长、单个果实重、果实开裂力度在3种环境尺度下种群间的差异均达到极显著水平, 而分枝数、果实长度的种群间变化不显著, 种群间的表型分化系数较低。不同环境尺度下株重、根重、单株果数均表现出一致的协变格局, 反映了生理功能性状之间整合对拟南芥适应环境的重要性。同时, 各种群间整体的性状协变差异性明显, 根长、单个果实重、分枝数、果实长度、果实开裂力度等特征与其他特征协变具有明显的局部性, 局域尺度和区域尺度环境之间的变化较大。聚类分析发现区域尺度上的不同种群聚合在一起的现象非常突出, 进一步表明拟南芥的表型特征受微环境的强烈影响。Mantel检验表明, 小尺度上10个种群株高、株重、根重、单个果实重、果实长度、果实开裂力度6个性状变化存在显著的空间相关性, 而分枝数、根长的相关性却不显著。因此, 我们认为拟南芥表型变化受小尺度环境的影响强烈, 但在表型层面并非所有性状都与原生境气候存在遗传关联。
SLR1 inhibits MOC1 degradation to coordinate tiller number and plant height in rice
DOI:10.1038/s41467-019-10667-2 URL [本文引用: 1]
“The integrative species concept” and “species on the speciation way”
DOI:10.17520/biods.2016222 URL [本文引用: 1]
“整合物种概念”和“分化路上的物种”
DOI:10.17520/biods.2016222
[本文引用: 1]

已有的各个物种概念对物种的认识类似盲人摸象, 只包含了物种的某一个方面; 而一个分化后期的成熟物种应涵盖了所有的物种概念。但是, 尚未到达分化后期的物种往往又已开始新一轮的物种分化; 自然中存在的多数“物种”处于分化路上。这种循环往复连续分化产生的物种, 存在种间生殖隔离不彻底、基因流频繁发生、网状进化突出等现象。此外, 对于不同的物种对, 最早开始分化的基因以及不同物种概念所要求的条件的分化顺序不是统一的, 而是随机的。定义一个适合所有“分化路上的物种”概念存在较大困难。但是, 应采用尽可能多的物种概念来界定分化路上的物种、发表新种和进行分类处理; 也应承认种间可能广泛存在不完全的生殖隔离和有限的基因流, 即有不属于两个物种群体的杂交或回交个体的存在。这样划分的物种比只依据一个物种概念认定的物种具有更高的客观性和科学性。
Phenotypic variation and covariation in natural populations of the exotic weedGaura parviflora in different habitat
不同生境小花山桃草自然种群表型变异与协变
On the concept of biodiversity
试论生物多样性的概念
Expansins: Roles in plant growth and potential applications in crop improvement
DOI:10.1007/s00299-016-1948-4 URL [本文引用: 1]
Which evolutionary processes influence natural genetic variation for phenotypic traits?
Transplantation of the subshrubLotus scoparius: Testing the home-site advantage hypothesis
DOI:10.1046/j.1523-1739.2000.99250.x URL [本文引用: 1]
Vegetative anatomy and systematics of subtribe Dendrobiinae (Orchidaceae)
DOI:10.1111/boj.1996.120.issue-2 URL [本文引用: 1]
Review of research onDendrobium, a prized folk medicine
DOI:10.1007/s00253-011-3829-7 URL [本文引用: 1]
Performance and variability patterns in wood properties and growth traits in the parents, F1 and F2 generation hybrid clones ofPopulus deltoides
DOI:10.1007/s11676-011-0182-8 URL [本文引用: 1]
B and C floral organ identity functions requireSEPALLATA MADS-box genes
DOI:10.1038/35012103 URL [本文引用: 1]
Phenotypic plasticity and evolution by genetic assimilation
DOI:10.1242/jeb.02070 URL [本文引用: 4]
Exploring phenotypic plasticity in the lichenRamalina capitata: Morphology, water relations and chlorophyll content in north- and south-facing populations
DOI:10.1006/anbo.1997.0453 URL [本文引用: 1]
Terrestrial Orchids:From Seed to Mycotrophic Plant
Testing for adaptation to climate inArabidopsis thaliana: A calibrated common garden approach
DOI:10.1093/aob/mcl282 URL [本文引用: 1]
Rapid auxin-induced cell expansion and gene expression: A four-decade-old question revisited
DOI:10.1104/pp.109.149591 PMID:20071604 [本文引用: 1]
Phenotypic plasticity of annual phlox: Tests of some hypotheses
DOI:10.1002/ajb2.1984.71.issue-2 URL [本文引用: 1]
Community functional responses to soil and climate at multiple spatial scales: When does intraspecific variation matter?
DOI:10.1371/journal.pone.0111189 URL [本文引用: 1]
The species concept
DOI:10.1111/evo.1951.5.issue-4 URL [本文引用: 1]
Pollinator-mediated selection on floral display, spur length and flowering phenology in the deceptive orchidDactylorhiza lapponica
DOI:10.1111/j.1469-8137.2010.03296.x
PMID:20497348
[本文引用: 1]

• Nonrewarding animal-pollinated plants commonly experience severe pollen limitation, which should result in strong selection on traits affecting the success of pollination. However, the importance of pollinators as selective agents on floral traits in deceptive species has not been quantified experimentally. • Here, we quantified pollinator-mediated selection (Δβ(poll)) on floral morphology and start of flowering in the deceptive orchid Dactylorhiza lapponica by subtracting estimates of selection gradients for plants receiving supplemental hand-pollination from estimates obtained for open-pollinated control plants. • There was directional selection for taller plants with more flowers and longer spurs, but no statistically significant selection on corolla size or flowering start. Pollinator-mediated selection accounted for all observed selection on spur length (Δβ(poll) = 0.32), 76% of the selection on plant height (Δβ(poll) = 0.19) and 42% of the selection on number of flowers (Δβ(poll = 0.30). Sixteen per cent of developing fruits were consumed by insect herbivores, but fruit herbivory had only minor effects on the strength of pollinator-mediated selection. • Our results demonstrate that pollinators mediate selection on floral traits likely to affect both pollinator attraction and pollination efficiency, and are consistent with the hypothesis that deceptive species experience strong selection for increased display and mechanical fit between flower and pollinator.© The Authors (2010). Journal compilation © New Phytologist Trust (2010).
Genetic characterization of a core collection of flax (Linum usitatissimum L.) suitable for association mapping studies and evidence of divergent selection between fiber and linseed types
DOI:10.1186/1471-2229-13-1 URL [本文引用: 2]
Phenotypic plasticity for plant development, function and life history
A single genotype can produce different phenotypes in different environments. This fundamental property of organisms is known as phenotypic plasticity. Recently, intensive study has shown that plants are plastic for a remarkable array of ecologically important traits, ranging from diverse aspects of morphology and physiology to anatomy, developmental and reproductive timing, breeding system, and offspring developmental patterns. Comparative, quantitative genetics and molecular approaches are leading to new insights into the adaptive nature of plasticity, its underlying mechanisms and its role in the ecological distribution and evolutionary diversification of plants.
Randomized response techniques for multiple sensitive attributes
DOI:10.1080/01621459.1981.10477741 URL [本文引用: 1]
A novel cytochrome P450 is implicated in brassinosteroid biosynthesis via the characterization of a rice dwarf mutant,dwarf11, with reduced seed length
DOI:10.1105/tpc.104.024950 URL [本文引用: 1]
A preliminary study of the orchid genusDendrobium SW China
中国石斛属的初步研究
Quantitative estimation of phenotypic plasticity: Bridging the gap between the evolutionary concept and its ecological applications
DOI:10.1111/jec.2006.94.issue-6 URL [本文引用: 1]
A novel gibberellin-induced gene from rice and its potential regulatory role in stem growth
Os-GRF1 (Oryza sativa-GROWTH-REGULATING FACTOR1) was identified in a search for genes that are differentially expressed in the intercalary meristem of deepwater rice (Oryza sativa L.) internodes in response to gibberellin (GA). Os-GRF1 displays general features of transcription factors, contains a functional nuclear localization signal, and has three regions with similarities to sequences in the database. One of these regions is similar to a protein interaction domain of SWI2/SNF2, which is a subunit of a chromatin-remodeling complex in yeast. The two other domains are novel and found only in plant proteins of unknown function. To study its role in plant growth, Os-GRF1 was expressed in Arabidopsis. Stem elongation of transformed plants was severely inhibited, and normal growth could not be recovered by the application of GA. Our results indicate that Os-GRF1 belongs to a novel class of plant proteins and may play a regulatory role in GA-induced stem elongation.
Genetic regulation of shoot architecture
DOI:10.1146/annurev-arplant-042817-040422 URL [本文引用: 1]
Research on phenotypic plasticity in plants: An overview of history, current status, and development trends
植物表型可塑性研究进展
Brassinosteroid signaling network and regulation of photomorphogenesis
DOI:10.1146/annurev-genet-102209-163450 URL [本文引用: 1]
Heritable and nonheritable relationships and variability of oil content and agronomic characters in the F2 generation of soybean crosses
DOI:10.2134/agronj1952.00021962004400040010x URL [本文引用: 1]
Biogeographical diversification of mainland AsianDendrobium (Orchidaceae) and its implications for the historical dynamics of evergreen broad-leaved forests
DOI:10.1111/jbi.12726 URL [本文引用: 2]
Molecular systematics ofDendrobium (Orchidaceae, Dendrobieae) from mainland Asia based on plastid and nuclear sequences
DOI:10.1016/j.ympev.2013.06.009 URL [本文引用: 4]
Evolution of Rosaceae fruit types based on nuclear phylogeny in the context of geological times and genome duplication
DOI:10.1093/molbev/msw242
PMID:27856652
[本文引用: 1]

Fruits are the defining feature of angiosperms, likely have contributed to angiosperm successes by protecting and dispersing seeds, and provide foods to humans and other animals, with many morphological types and important ecological and agricultural implications. Rosaceae is a family with ∼3000 species and an extraordinary spectrum of distinct fruits, including fleshy peach, apple, and strawberry prized by their consumers, as well as dry achenetum and follicetum with features facilitating seed dispersal, excellent for studying fruit evolution. To address Rosaceae fruit evolution and other questions, we generated 125 new transcriptomic and genomic datasets and identified hundreds of nuclear genes to reconstruct a well-resolved Rosaceae phylogeny with highly supported monophyly of all subfamilies and tribes. Molecular clock analysis revealed an estimated age of ∼101.6 Ma for crown Rosaceae and divergence times of tribes and genera, providing a geological and climate context for fruit evolution. Phylogenomic analysis yielded strong evidence for numerous whole genome duplications (WGDs), supporting the hypothesis that the apple tribe had a WGD and revealing another one shared by fleshy fruit-bearing members of this tribe, with moderate support for WGDs in the peach tribe and other groups. Ancestral character reconstruction for fruit types supports independent origins of fleshy fruits from dry-fruit ancestors, including the evolution of drupes (e.g., peach) and pomes (e.g., apple) from follicetum, and drupetum (raspberry and blackberry) from achenetum. We propose that WGDs and environmental factors, including animals, contributed to the evolution of the many fruits in Rosaceae, which provide a foundation for understanding fruit evolution.© The Author 2016. Published by Oxford University Press on behalf of the Society for Molecular Biology and Evolution.
A study on morphological variation of different populations of Lespedeza davurica
不同居群达乌里胡枝子形态变异研究
Molecular Phylogeny of Dendrobium and Genome-wide Analysis of NBS Genes in D. catenatum
石斛属的系统发育和铁皮石斛NBS基因的分析
Comments on species-level taxonomy of plants in China
DOI:10.17520/biods.2016226 URL [本文引用: 1]
我国植物种级水平分类学研究刍议
DOI:10.17520/biods.2016226
[本文引用: 1]

对洪德元先生最近在《生物多样性》(2016年第24卷第3期)发表的《关于提高物种划分合理性的意见》一文中的部分观点进行了进一步阐述。强调我国植物确实还存在大量种级水平的分类学问题有待解决, 我国植物分类学研究在一些重要发展阶段(如系统阶段和物种生物学阶段)上存在明显缺失, 需要弥补。指出分类学发展到今天, 不宜再强调“经典分类学”和“实验分类学”之分, 应采用多学科手段解决分类学问题; 我国应加强植物分类专著水平的研究工作, 注意培养年轻一辈植物分类学专著工作者; 在分类处理中应用居群概念和统计学方法时应特别谨慎; 在系统植物学中接受物种概念的多元性是必要的, 但要向达到广义的生物学种概念努力, 不宜以有所谓的“归并派”和“细分派”之分为借口而完全主观地划分物种。
Vegetative diversification and radiation in subtribe Dendrobiinae (Orchidaceae): Evidence from chloroplast DNA phylogeny and anatomical characters
Phylogenetic relationships among A-genome species of the genusOryza revealed by intron sequences of four nuclear genes
DOI:10.1111/j.1469-8137.2005.01406.x URL [本文引用: 2]