互利共生或者互惠关系在自然界中非常普遍, 涉及的物种遍布自然界中的所有门类, 几乎地球上的每一个物种都处于一种甚至多种互利互惠关系中(Bronstein, 2001)。植物与传粉昆虫形成的互利互惠关系是一种典型且非常普遍的互利共生关系。其中, 榕树(桑科榕属Ficus)及其传粉榕小蜂是目前所知道的自然界中关系最为紧密的互利合作关系之一(Weiblen, 2002; Yang et al, 2004; Machado et al, 2005)。在二者的互利共生关系中, 榕树为榕小蜂提供抚养后代的营养以及繁殖的场所, 榕小蜂为榕树传粉, 帮助榕树完成繁殖(Janzen, 1979; Weiblen, 2002; Cook & West, 2005)。
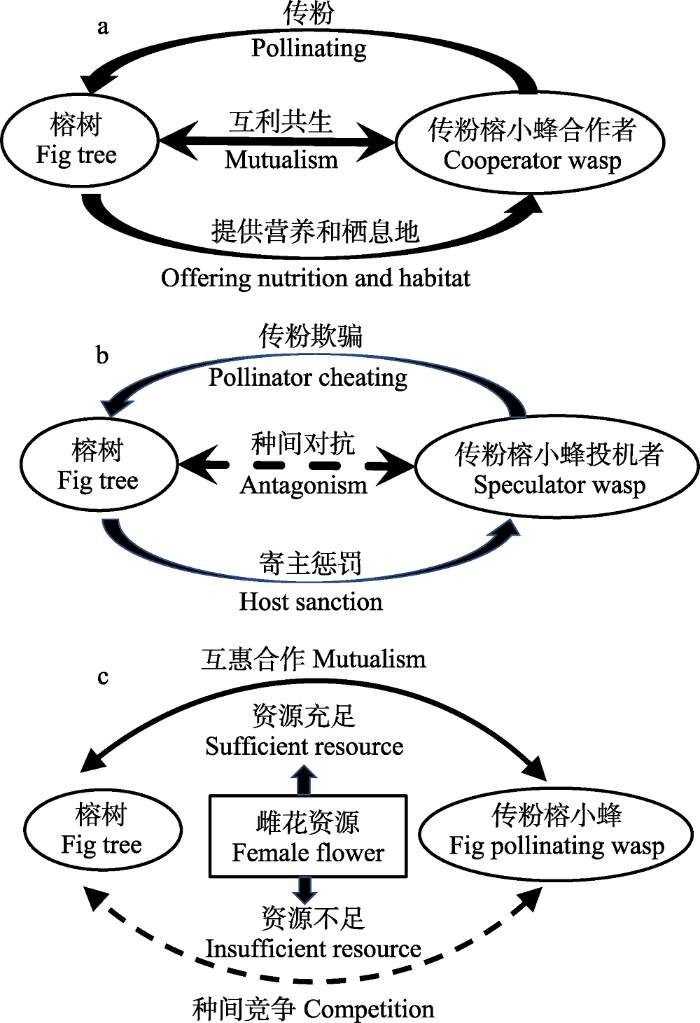
榕树与其传粉榕小蜂之间的协同进化历史长达几百万年(Chen et al, 2010; Cruaud et al, 2012), 在长期的协同进化过程中, 二者之间在保持互惠合作的同时, 也存在着竞争等对抗关系。在榕树及其传粉榕小蜂的相互作用关系中, 对雌花资源的利用、分配以及榕小蜂的传粉行为是二者合作的基础, 也是竞争的焦点。榕小蜂为榕树传粉, 榕树为榕小蜂提供营养和栖息地, 二者之间表现出合作关系(图1a); 在榕小蜂不携带花粉的情况下, 榕树会对榕小蜂进行惩罚, 二者之间形成对抗关系(图1b)。在雌花资源充足的情况下, 榕树与传粉榕小蜂呈现出互惠合作关系; 但是在雌花资源被双方过度利用, 雌花资源不足的情况下, 二者之间会针对雌花资源产生竞争(图1c)。
图1
图1
榕树及其传粉榕小蜂之间的相互作用关系图。a, 榕小蜂为榕树传粉, 榕树为榕小蜂提供营养和栖息地, 二者之间表现为互利共生关系; b, 传粉榕小蜂投机者不执行传粉功能, 但是仍在利用榕树的资源, 榕树对投机者进行寄主惩罚, 二者之间形成对抗关系。c, 在雌花资源充足与否的两种情况下, 榕树与传粉榕小蜂表现出合作关系或者竞争关系。本图根据Wang等(2011)整理。
Fig. 1
The interaction between figs and their fig pollinating wasps. a, the mutualism was based on figs offering nutrition and habitat to the wasp and the wasps pollinating the figs; b, pollination cheating and host sanction lead to antagonism relationship between fig and fig pollinating wasp; c, cooperation or competition between fig and fig pollinating wasp was dependent on the supply of common resource (according to Wang et al, 2011).
相比于对称性竞争关系, 自然界中存在大量的非对称性竞争关系: 比如动物竞争中, 存在着双方体型大小、力量强弱的差异, 资源的拥有者和非拥有者之间的差异等。竞争关系中双方不对称的地位会对竞争的结果产生影响(Smith, 1982; 张大勇, 2000)。榕树是雌花资源的提供者, 使得榕树与榕小蜂之间对雌花资源的竞争形成非对称性竞争关系。在雌花资源的利用、分配以及传粉行为的博弈过程中, 二者之间呈现出实力不对称和信息不对称。在对雌花资源的利用和分配中, 榕树掌握着雌花资源(Murray, 1985), 使得榕树在与榕小蜂的种群密度依赖竞争中占据了优势地位(Yu et al, 2004; Wang et al, 2011)。在传粉行为的博弈过程中, 虽然在传粉榕小蜂不执行传粉行为的情况下, 榕树个体内部的资源分配能够减少甚至不提供营养给这些榕小蜂(Peng et al, 2008; Jander & Herre, 2010), 但是不传粉的投机者能够在传粉者个体的掩护下逃避榕树的惩罚。榕树并不能够准确地判断特定的某个榕小蜂是否传粉, 其寄主惩罚的效力因此而打折扣, 使得不传粉的投机者个体能够在种群中保存下来(Jander & Herre, 2016)。
在榕树与传粉榕小蜂协同进化过程中, 二者世代更迭速度不同、进化速率不对称影响了它们的进化历程以及相互对抗关系中的博弈策略。由于榕小蜂的世代较短, 世代更迭速度和进化速率较快(Frankham, 1996; Souto-Vilarós et al, 2019), 短时间内积累了更多的基因突变(Herre et al, 1999), 为榕小蜂的行为多样性提供了物质基础。对于传粉榕小蜂来说, 与榕树的博弈是单次的、不重复的, 完成一次传粉和产卵, 亲本小蜂就结束了使命和生命(Weiblen, 2002), 其策略的改变只能通过代际之间的遗传突变来完成; 而对于榕树来说, 一棵榕树与传粉榕小蜂之间的博弈是多次的, 每一季榕树的策略或形态特征都可以发生变化, 其策略的变化通过表型可塑性来实现。由于进化速率的不对称, 榕树与传粉榕小蜂之间的博弈策略演变的物质基础不同, 策略多样性的产生方式也不同。
榕树与传粉榕小蜂之间合作与竞争的关系随着条件和环境的变化而转变(Wang et al, 2011, 2015b)。公共资源(雌花)是否充足, 会影响资源分配过程中榕树和小蜂之间的相互作用关系。榕树和传粉榕小蜂之间的非对称性关系导致二者后代适合度收益存在不确定性, 促进二者博弈策略和行为的多样性。在长期的协同进化过程中, 榕树和榕小蜂在竞争博弈过程中的行为多样性将可能在基因水平上固定下来, 促进遗传多样性及物种多样性的形成。本文将着重探讨榕树与传粉榕小蜂相互作用关系中的实力不对称、信息不对称以及进化速率不对称, 及其对榕树榕小蜂互利共生关系的影响。
1 种间竞争中的实力不对称
即使是同一个体的基因组上也存在着不同基因之间的利益冲突, 互利共生系统内部的相互作用关系也不是绝对和谐的(Herre et al, 1999)。榕树与传粉榕小蜂之间潜在的利益冲突主要集中在繁殖上对彼此的依赖。榕小蜂繁殖后代的营养全部来自于榕果, 榕树可以通过控制榕果的进蜂量来控制榕小蜂的种群密度(Yu et al, 2004; Wang et al, 2011); 雌花资源分配会影响榕树和榕小蜂的繁殖成功率, 对雌花的竞争更是形成了产卵器与花柱长度之间的军备竞赛(Weiblen, 2004); 榕小蜂的传粉行为是榕树繁殖的保障, 榕树对于榕小蜂传粉成功率(诱骗小蜂为雌树传粉) (Weiblen, 2002; Cook & Rasplus, 2003), 传粉的忠诚度(惩罚欺骗者) (Guan et al, 2007)以及传粉效率(从主动传粉中收益) (Kjellberg et al, 2001)方面, 都形成了相应的对策。在这一系列的冲突中, 榕树是资源的提供者和控制者, 在冲突中享有更高的权利。
1.1 种群密度依赖的种间竞争
榕树与传粉榕小蜂的种群密度具有很大差异, 一株榕树一季可以结出成百上千个榕果, 每个榕果内可以养育几十到数千头小蜂。在榕树-传粉榕小蜂之间的密度依赖的种间竞争中, 榕树是优势方(Yu et al, 2004; Wang et al, 2011)。榕树通过限制进入榕果的小蜂数量来控制传粉榕小蜂对雌花资源的利用, 从而调节传粉榕小蜂的种群数量, 以达到调控种间竞争强度的目的。
隐头花序是榕属植物的特有特征。隐头花序的结构限制了榕树传粉者的种类, 使得只有专一性的传粉榕小蜂才能够为榕树传粉。隐头花序的结构也便于榕树对传粉者的控制, 尤其是对传粉者种群密度的控制(Yu et al, 2004; Wang et al, 2011)。在榕果接受期, 在没有榕小蜂及时访问并进入榕果(隐头花序)的情况下, 榕果能够保持数天的接受期状态, 某些榕树物种如聚果榕(F. racemosa)、鸡嗉子榕(F. semicordata)的苞片口会张大以便传粉榕小蜂进入。但是如果有多只蜂短时间内进入榕果, 苞片口会迅速闭合, 进入榕果的通道迅速封紧(Wang et al, 2009, 2015a; 张媛等, 2014)。同时, 榕树还会通过改变挥发性物质的成分来吸引或者排斥传粉榕小蜂。当很少或者没有榕小蜂进入榕果时, 榕树会释放出挥发物来吸引传粉榕小蜂; 当有足够的榕小蜂进入榕果后, 挥发物的内容和含量会发生改变, 不再吸引甚至拒绝传粉榕小蜂靠近(Proffit et al, 2008; Chen et al, 2009; Gu et al, 2012)。
传粉榕小蜂后代的性比率的改变也能起到间接调控其种群密度的效果(Zhang et al, 2004)。传粉榕小蜂是单双倍型昆虫, 亲本雌蜂通过控制卵子是否受精来控制后代的性比。在进蜂量较低的情况下, 小蜂后代近亲交配的概率更高, 小蜂倾向于产生更加偏雌的性比率(Herre, 1985, 1987), 雌性个体数量更多能够使得种群更容易扩大。进蜂量影响榕小蜂近交水平进而影响性比率的调节过程需要连续几个世代才能产生调节种群大小的效果。在密度依赖的种间竞争过程中, 榕树通过控制进蜂量直接调控小蜂的种群数量; 而传粉榕小蜂仅能通过调控后代性比率来间接调控种群大小。
1.2 雌花资源竞争中的军备竞赛
榕树与传粉榕小蜂之间对雌花的竞争表现出对抗性和浪费性(Murray, 1985), 其中传粉榕小蜂因军备竞赛而导致的产卵器长度浪费性增长, 在雌雄同株榕树的传粉榕小蜂中是能够被观察和检测到的(Weiblen, 2004)。
榕树的隐头花序是传粉榕小蜂抚育后代的唯一场所(Janzen, 1979; Cook & West, 2005)。传粉榕小蜂将产卵器通过榕树的花柱插入雌花的子房, 小蜂的卵在雌花的子房内发育成长(Janzen, 1979; Weiblen, 2002)。只有当产卵器长度比花柱长的情况下, 小蜂的产卵器才能到达子房, 进而成功产卵。榕树的雌花不能同时既抚育小蜂又产生种子, 所以榕树和小蜂在雌花的利用上形成资源竞争(Weiblen, 2002; Wang et al, 2015a)。在雌雄异株的榕树中, 雌树的雌花全部产生种子, 不养育小蜂, 而雄树的雌花全部养育小蜂, 不产生种子, 榕树和传粉小蜂对榕树雌花利用不存在竞争。但是在雌雄同株榕树中, 小蜂和种子在同一个隐头花序内发育, 榕树和传粉小蜂对榕树雌花利用中存在竞争(表1)。相比于雌雄异株榕树的传粉小蜂, 雌雄同株榕树的传粉榕小蜂经历了花柱长度和产卵器长度之间的军备竞赛, 因而进化出过长的产卵器。Weiblen (2004)曾经测量了43种传粉榕小蜂, 发现雌雄异株榕树的传粉小蜂的产卵器都比较短(28种), 而80% (12种)的雌雄同株榕树的传粉小蜂的产卵器都比较长(表2)。
表1 榕树隐头花序和小花的性别功能分配
Table 1
| 雌雄同株 Monoecy | 雌雄异株 Dioecy | 性别功能 Sexual function | ||
|---|---|---|---|---|
| 雄树 Male tree | 雌树 Female tree | |||
| 雄花发育产物 Product from male flower | 花粉 Pollen | 花粉 Pollen | - | 雄性功能 Male function |
| 雌花发育产物 Product from female flower | 小蜂 Wasp | 小蜂 Wasp | - | |
| 种子 Seed | - | 种子 Seed | 雌性功能 Female function | |
表2 不同的寄主榕树性系统与其传粉榕小蜂产卵器长度的对应关系
Table 2
| 寄主榕树性系统 Host sexual system | 产卵器长度大于腹部的传粉小蜂种数 Ovipositor is longer than the thorax | 产卵器长度短于腹部的传粉小蜂种数 Ovipositor is shorter than the thorax | 总计 Total |
|---|---|---|---|
| 雌雄同株 Monoecy | 12 | 3 | 15 |
| 雌雄异株 Dioecy | 0 | 28 | 28 |
| 总计 Total | 12 | 31 | 43 |
数据来自
延长花柱长度与延长产卵器长度所付出的代价差异巨大。对于榕树来说, 对花柱生长进行的营养投资只占繁育投资中很小的份额, 但是对于传粉榕小蜂来说, 过长的产卵器给飞行带来了巨大的负担。Jander等(2016)发现, 榕小蜂的体型大小对榕小蜂能否顺利到达榕果的影响很大。对于传粉榕小蜂来说, 资源过度投入到产卵器生长上, 显然是一个巨大的负担。在竞争过程中, 传粉榕小蜂进化出更长的产卵器以便在更多的雌花中产卵(Ganeshaiah et al, 1995; Weiblen, 2004; Ghana et al, 2017); 而榕树通过增长花柱长度限制传粉榕小蜂产卵, 保证了种子对雌花资源的利用。
在种群/物种水平上, 榕树与其传粉榕小蜂之间在花柱和产卵器长度上存在军备竞赛; 在不同物种的水平上, 花柱和产卵器长度之间存在着精确的匹配关系(Weiblen, 2004)。花柱和产卵器长度之间精确的共适应, 可能恰恰是在激烈的军备竞赛之下权衡的结果。
1.3 主动传粉行为给榕树带来的适合度收益
榕小蜂是榕树忠诚而高效的传粉者。传粉榕小蜂形态、行为的特化使其能够高效特异地将花粉传给寄主榕树。很多传粉榕小蜂物种具有花粉筐、花粉梳等结构, 具有主动收集和散播花粉的行为; 而另一部分传粉榕小蜂物种则通过身体粘黏携带花粉, 完成散粉(Cook & Lopez-Vaamonde, 传粉榕小蜂的主动传粉行为给榕树带来的最直接的收益就是节约花粉。针对88种传粉榕小蜂及其寄主榕树的调查发现(Kjellberg et al, 2001), 在传粉者主动传粉的情况下, 榕树的花粉(花药)数量较少, 其花药胚珠比(anther : ovule)小于0.16, 而不具有主动传粉者的榕树, 其花药胚珠比(anther : ovule)在0.21以上(Kjellberg et al, 2001; Cook & Rasplus, 2003)。
对于传粉榕小蜂来说, 主动传粉行为的收益并不很明确(Kjellberg et al, 2001; Jousselin et al, 2003)。缺少花粉会导致传粉榕小蜂的后代数量和质量下降(Jousselin et al, 2003; Wang et al, 2014b; Jander et al, 2016; Jander & Herre, 2016); 另一方面, 在物种水平上, 传粉榕小蜂彻底放弃主动传粉行为似乎并没有太大的损失, 在被动传粉的榕小蜂物种中, 花粉缺失似乎对榕小蜂后代的发育影响不大(Peng et al, 2008; 徐睿等, 2016), 传粉小蜂并不会因为不携带花粉而受到寄主榕树的惩罚, 被动传粉的榕小蜂也可以在未授粉的雌花中发育(Kjellberg et al, 2001; Jousselin et al, 2003)。
1.4 榕树通过寄主惩罚控制欺骗者的出现
主动传粉能够给榕树带来收益, 同时榕树需要通过额外的手段来阻止不传粉的欺骗者的出现。榕树可以通过3种方式来惩罚不传粉的榕小蜂: (1)落果, 直接将全部小蜂后代杀死(Guan et al, 2007; Jander et al, 2012; Wang et al, 2014a; Zhang XW, 2019); (2)减少小蜂后代的数量(Jander & Herre, 2010; Jander et al, 2012); (3)降低小蜂后代的质量(Jander et al, 2016)。寄主惩罚的方式可能是有效的, 针对巴拿马4种传粉榕小蜂的调查发现, 寄主惩罚的强度越强, 种群中出现不携带花粉的个体(欺骗者)的概率就越低(Jander & Herre, 2010)。并且榕树与传粉榕小蜂之间互利共生关系的紧密程度会影响榕树对小蜂的惩罚强度: 关系越紧密, 惩罚强度越弱(Wang et al, 2015a)。榕树所实施的寄主惩罚效应, 有效减少了欺骗者小蜂的数量。在种群水平上, 寄主惩罚效应是维持榕树-传粉榕小蜂互利共生关系的有力手段。
2 信息不对称
在榕树与传粉榕小蜂的竞争博弈过程中, 由于彼此的信息不对称而发生了诸多欺骗事件, 使得榕树与传粉榕小蜂之间在个体水平上的博弈策略变得更加丰富多样。
2.1 雌树对传粉者的诱骗
榕小蜂为榕树传粉是榕树-传粉榕小蜂之间互惠合作的基础, 但在雌雄异株榕树中, 雄树抚育小蜂, 雌树生产种子, 进入雌树榕果的小蜂不能产生后代, 雌树的榕果对于传粉榕小蜂来说是一个死亡陷阱(Weiblen, 2002; Cook & Rasplus, 2003)。所以选择压力使得传粉榕小蜂倾向于分辨雌树和雄树, 找到与出生时一样的树(雄树)来完成繁育后代。有证据表明雌雄异株榕树的传粉榕小蜂对寄主榕树的识别能力更强, 识别错误率更低(Yang et al, 2015)。然而, 雌树通过模仿雄树的挥发物, 仍然能够成功吸引到传粉小蜂(Soler et al, 2012; Hossaert-McKey et al, 2016), 传粉榕小蜂仍然不能避免进入雌树的榕果, 为榕树传粉却不能完成自己的适合度(Hossaert-McKey et al, 2010; Gu et al, 2012; Soler et al, 2012); 但是小蜂保证了榕树种群的繁衍, 也就是保证了小蜂繁殖后代所需的栖息地和营养来源。
2.2 榕小蜂的传粉欺骗
传粉榕小蜂在为榕树传粉的过程中并不总是忠诚的, 不传粉的欺骗者以一定比例在种群中存在(Jander & Herre, 2010), 甚至存在传粉榕小蜂进化成为欺骗者, 即稳定的不传粉的物种的现象(Galil & Eisikowitch, 1968; Compton et al, 1991; Peng et al, 2008; Zhang T et al, 2019)。对于传粉榕小蜂来说, 逃避传粉是有益处的, 因为携带花粉飞行需要耗费体力, 只有体型较大的小蜂才能成功到达目的地(接受期榕果), 然而由于榕果苞片口的特殊结构, 阻止了体型过大的小蜂进入榕果, 使得无论是过大或者过小的传粉者个体都不能成功到达并进入榕果。在对高榕(Ficus altissima)的调查发现, 到达榕果的传粉榕小蜂Eupristina. altissima明显比欺骗者Eupristina sp.的体型大, 而进入榕果的传粉榕小蜂却明显比欺骗者的体型小(Xu et al, 2016)。并且, 欺骗者榕小蜂似乎并没有因为不携带花粉而受到惩罚, 其后代的适合度并没有比传粉者低(Peng et al, 2008)。
不携带花粉的欺骗者能够在榕树-传粉榕小蜂相互作用关系的博弈中找到有利的机会。西双版纳地区的小叶榕(Ficus microcarpa)的传粉欺骗者似乎利用了传粉者种群密度季节性变动的特点, 在传粉者数量较少的季节大量利用榕树的闲置资源来完成繁殖(Zhang T et al, 2019)。欺骗者似乎在互惠关系更紧密的榕树-传粉榕小蜂系统中更容易出现。例如在巴拿马地区, 主动传粉的榕小蜂种群内存在欺骗者, 而被动传粉的榕小蜂种群内反而没有欺骗者(Jander & Herre, 2010)。目前发现的3种欺骗者传粉小蜂, 在高榕和小叶榕中的进蜂量都在1-2头/果(Ficus sycomorus的进蜂量无数据), 进蜂量较小被认为是榕树与传粉榕小蜂互惠关系更紧密的特征。这暗示了榕树与榕小蜂在相互依赖程度更高时, 彼此之间的博弈更容易发生多样化的策略。
2.3 寄主惩罚的局限性
虽然榕树对传粉欺骗行为有一定的惩罚措施, 但是寄主惩罚的精确性却很差: 当携带花粉的小蜂(合作者)和不携带花粉的小蜂(欺骗者)进入同一个榕果内产卵的时候, 榕树不能够进行区分, 惩罚措施只能实施在整个榕果上(Jander et al, 2016), 这使得不携带花粉的个体能够在合作者的掩护下繁育后代, 完成适合度收益。在这种信息不对称的情况下, 欺骗者得以在种群中以一定的比例存在。在单果进蜂量更大的物种中, 投机者更容易得到传粉者的掩护, 这可能解释了为什么单果进蜂量大的榕树对投机者的惩罚强度更强(Wang et al, 2015a)。
雌雄异株榕树的雌树和雄树的性别功能不同, 雄树主要承担养育传粉者、散播花粉的功能(表1)。对于雄树来说, 进入榕果的小蜂是否携带花粉并无影响; 影响雄树适合度的是子代小蜂离开榕果时是否会收集和携带花粉。有调查发现, 同一物种的雄树对不携带花粉的小蜂要比雌树宽容得多(Zhang T et al, 2019)。这就给不携带花粉的小蜂提供了在种群中保留下来的机会。
3 榕树与传粉榕小蜂的进化速率不对称
榕树与其传粉榕小蜂之间有着紧密的互利共生关系和严格的协同进化关系(Cruaud et al, 2012), 可能促进进化速率的加快。针对Pseudomyrmex属蚂蚁的比较基因组研究发现, 在亲缘关系较近的不同种蚂蚁之间, 有共生习性的蚂蚁比没有共生性的蚂蚁的进化速率要高(Rubin & Moreau, 2016); 而榕属的物种多样性也明显比桑科的其他属高, 桑科有40多个属, 共1,000多种, 其中榕属就有750多种(Moe et al, 2012)。“红皇后假说”非常形象地解释了互利共生关系加快进化速率的现象。红皇后假说来源于《爱丽丝梦游仙境》中红皇后对爱丽丝说的名言“你只有拼命奔跑, 才能够原地不动”。在持续的选择压力下, 互利共生的双方会形成进化上的军备竞赛, 导致双方都尽可能快地进化(Herre et al, 1999; Thompson, 2013)。榕树与传粉榕小蜂之间紧密的互利共生关系促进了二者的协同进化, 甚至协同成种, 进而加速了二者的成种速率。
在榕树与传粉榕小蜂的协同进化过程中, 两者进化速率差异很大。榕树为木本植物, 包括高大乔木和灌木以及藤本植物, 寿命多在十几甚至上百年(Berg et al, 2005), 然而传粉榕小蜂的寿命通常仅1-2个月(Weiblen, 2002)。榕树与传粉榕小蜂的世代周期长度差别很大: 传粉榕小蜂一年可以经历几个世代, 然而榕树的种子长成大树, 再到开花结果则需要数年的时间。世代时间影响进化速率(Sharp & Li, 1989), 榕小蜂的基因突变率也比榕树更高(Frankham, 1996; Cook & Rasplus, 2003), 加之严重的近交因素等使榕小蜂的物种分化往往快于榕树(Herre et al, 2008; Chen et al, 2010), 这就导致了榕小蜂的遗传多样性比榕树高(Souto-Vilarós et al, 2019)。例如, 频繁发现的传粉榕小蜂隐种事件暗示了传粉榕小蜂的物种数可能要比榕树更多(Molbo et al, 2003; Haine et al, 2006; Huang et al, 2018) 。
榕树与榕小蜂之间进化速率不对称使得二者在博弈过程中使用的策略不同, 榕小蜂的行为多样性以遗传突变为基础, 而榕树则通过表型可塑性来产生策略的多样性。例如榕小蜂的传粉欺骗行为在种群中固定下来可以通过遗传突变来解释, 而榕树则通过营养资源重新分配来完成对传粉榕小蜂的惩罚(Jander & Herre, 2016)。Jander和Steidinger (2017)通过模型模拟分析发现, 巴拿马地区的4种传粉榕小蜂种群中稳定存在的欺骗者小蜂可能就是突变产生的。更进一步, 欺骗行为的固定甚至可以促进物种的分化, 已经分化成种的欺骗者传粉小蜂物种也被证实是由传粉榕小蜂经物种复制分化(speciation duplication)而来(Yang et al, 2015; Zhang T et al, 2019)。榕树对于欺骗者的惩罚通过停止或减少对欺骗者后代的营养投入来实现(Jander & Herre, 2016)。在整个榕果没有进入携带花粉小蜂(只有欺骗者进入)的情况下, 榕果内没有种子形成, 榕树会放弃整个榕果(落果), 榕树的营养资源将重新分配给有花粉的(进入传粉者的)榕果, 以保证榕树的种子产量(Guan et al, 2007; Zhang T et al, 2019)。对于同时有欺骗者和传粉者进入的榕果, 榕果的种子较少, 榕树对榕果投入资源量也减少, 导致传粉榕小蜂后代数量和质量降低(Jander & Herre, 2010; Jander et al, 2012)。榕树甚至能够精确地判断虫瘿的性别, 分配给雌性幼虫比雄性幼虫更多的营养(Li et al, 2016)。
榕树花柱长度与传粉榕小蜂产卵器长度之间的博弈也受到进化速率不对称的影响。对聚果榕的研究发现, 随着纬度的变化, 花柱长度以及传粉榕小蜂的产卵器长度也发生相应的变化(王瑞武等, 未发表数据)。其中花柱长度的变化更可能是由于环境作用导致的表型可塑性变化, 而榕小蜂的产卵器长度的变异更可能是来源于遗传多样性。
4 总结
在榕树与传粉榕小蜂之间长期的协同进化历史中, 除了合作互惠关系之外也存在对资源的竞争、对抗关系。榕树-传粉榕小蜂之间紧密的互惠合作关系和多样的博弈策略, 使得榕树-传粉榕小蜂系统成为研究竞争、对抗关系的最佳模型。由于榕树与传粉榕小蜂之间的实力不对称和信息不对称, 使得二者在资源竞争、传粉行为冲突中表现出丰富多样的策略(表3)。由于进化速率不对称, 榕树与传粉榕小蜂博弈策略产生的来源和物质基础不同, 榕树通过表型可塑性产生的形态变异与传粉榕小蜂由于遗传多样性而产生的行为多样性之间发生博弈。在二者的竞争关系中, 在不同的博弈策略下, 榕树与榕小蜂的适合度收益也不同。在榕树与传粉榕小蜂的竞争博弈中, 公共资源(雌花)在榕树种子发育和小蜂后代抚育的分配中存在不确定性, 导致适合度收益存在不确定性。榕树与榕小蜂丰富多样的形态特征和行为策略在基因水平固定下来, 促进了种群遗传多样性的发生, 甚至形成物种分化, 促进了物种多样性的形成。
表3 榕树-榕小蜂在传粉行为中的非对称关系
Table 3
| 博弈策略 Strategy | 受益方 Winner | 博弈结果 Result | 非对称关系 Asymmetric interaction | 代表性文献 Typical reference |
|---|---|---|---|---|
| 主动传粉 Active pollinating | 榕树 Figs | 榕树节约花粉 Figs produce less pollens | 实力不对称 Asymmetric power | Kjellberg et al, 2001 |
| 传粉欺骗 Pollinator cheating | 小蜂 Wasps | 小蜂减少传粉负担 Wasp takes less burden for carrying pollens | 信息不对称 Asymmetric information | Peng et al, 2008 |
| 寄主惩罚 Host sanction | 榕树 Figs | 防止欺骗者的出现 Reducing the cheating wasps | 实力不对称 Asymmetric power | Jander & Herre, 2010 |
| 传粉投机者 Speculator | 小蜂 Wasps | 小蜂逃避寄主惩罚 The wasps avoid the host sanction | 信息不对称 Asymmetric information | Jander et al, 2012 |
| 雌树诱骗 Female tree attracts wasp by mimicking male tree | 榕树 Figs | 小蜂失去繁殖机会 Wasps fail to reproduce | 实力不对称 Asymmetric power | Cook & Rasplus, 2003 |
| 基因突变产生欺骗者 Mutation causes cheaters | 小蜂 Wasps | 维持种群内投机者比例 Stable proportion of cheaters in population | 进化速率不对称 Asymmetric evolution rates | Jander & Steidinger, 2017 |
参考文献
Flora Malesiana (Moraceae-Ficus)
The exploitation of mutualisms
DOI:10.1046/j.1461-0248.2001.00218.x URL [本文引用: 1]
Private channel: A single unusual compound assures specific pollinator attraction in Ficus semicordata
DOI:10.1111/fec.2009.23.issue-5 URL [本文引用: 1]
Species-specificity and coevolution of figs and their pollinating wasps
Mutualism is one of the most important ecological interactions, with strong influences on almost all levels of biological systems. Their long-term persistence raises many challenging evolutionary questions, especially those involving high-level coevolution and coadaptation. Figs and their pollinating wasps are among the most tightly integrated mutualisms known, and provide a model system for developing and testing theories of coevolution. Initial studies suggested specific coevolution between them, described as the famous rule of one fig one wasp. However, more and more exceptions have been revealed by recent studies, and cryptic species in pollinating wasps and host switching were found common in some regions and within some Ficus groups, inducing debates on the levels of species specificity and coevolution. A broad-sense coevolution model to describe the relationship of the related groups of figs and their pollinating wasps was proposed recently. The diverse relationships between figs and their pollinating wasps indicated coexistence of both specific and diffuse coevolution in this mutualism system, producing different species-specificity level. However, which model is the dominant one in this system is still keeping open. The species specificity could be tight or loose in different regions and fig groups involved. Consequently, the frequencies and mechanisms of breakdowns of the one-to-one rule within different fig groups as well as in different regions are essential for the understanding of the relative importance of the competing finer-scale cospeciation or broad-sense co-evolution models.
榕-传粉榕小蜂间的专一性与协同进化
Studies of Ceratosolen galili, a non-pollinating agaonid fig wasp
DOI:10.2307/2388305 URL [本文引用: 1]
Fig biology: Turning over new leaves
DOI:10.1016/S0169-5347(00)02038-3 URL
Mutualists with attitude: Coevolving fig wasps and figs
DOI:10.1016/S0169-5347(03)00062-4 URL [本文引用: 5]
Figs and fig wasps
DOI:10.1016/j.cub.2005.11.057 URL PMID:16360672 [本文引用: 2]
An extreme case of plant-insect co-diversification: Figs and fig-pollinating wasps
Relationship of genetic variation to population size in wildlife
On the pollination ecology of Ficus sycomorus in East Africa
Evolution of style-length variability in figs and optimization of ovipositor length in their pollinatior wasps: A coevolutionary model
Style length variation in male and female figs: Development, inheritance, and control of pollinator oviposition
DOI:10.1111/eea.12533 URL [本文引用: 1]
“Push” and “pull” responses by fig wasps to volatiles released by their host figs
DOI:10.1007/s00049-012-0108-8
URL
[本文引用: 2]

In the specific mutualism between fig trees (Ficus) and their obligate pollinating fig wasps (Agaonidae), it is crucial that fig wasps can recognize the developmental stages of their host figs. However, the responses of fig wasps to volatiles released from figs during their developmental phases are less clearly understood and are the focus of this study. We extracted and identified the volatiles released from the figs of Ficus curtipes throughout their development. Using Y-tube choice experiments, we also compared the behavioural responses of the tree's pollinator (Eupristina sp.) to figs at different developmental stages, and compared these results to those obtained by trapping fig wasps as they arrived at a tree with a developing fig crop. The chemical composition of the fig volatiles changed during fig development with the blends exhibiting clear segregation among figs at different developmental phases. Male phase figs had the most distinct blend. Fig wasp females were preferentially attracted to receptive figs, but figs at most other developmental phases were also attractive. Conversely, male phase figs had a repellent effect. These results were supported by the behaviour of the wasps under natural conditions, with small numbers of fig wasps arriving at the tree before and after receptive figs were present. These results indicate a more complex relationship between fig volatiles and fig wasp behaviour than previously realized, with volatiles mediating both the initial meeting of the mutualists to achieve pollination and egg laying and the subsequent departure of the next generation of fig wasps. This offers an explanation for the specialization and long-term coexistence of figs and fig wasps.
Host sanctions in fig-fig wasp mutualism
榕-蜂互惠关系中榕树对未传粉榕小蜂的惩罚效应
Deep mtDNA divergences indicate cryptic species in a fig-pollinating wasp
DOI:10.1186/1471-2148-6-83
URL
PMID:17040562
[本文引用: 1]

BACKGROUND: Figs and fig-pollinating wasps are obligate mutualists that have coevolved for ca. 90 million years. They have radiated together, but do not show strict cospeciation. In particular, it is now clear that many fig species host two wasp species, so there is more wasp speciation than fig speciation. However, little is known about how fig wasps speciate. RESULTS: We studied variation in 71 fig-pollinating wasps from across the large geographic range of Ficus rubiginosa in Australia. All wasps sampled belong to one morphological species (Pleistodontes imperialis), but we found four deep mtDNA clades that differed from each other by 9-17% nucleotides. As these genetic distances exceed those normally found within species and overlap those (10-26%) found between morphologically distinct Pleistodontes species, they strongly suggest cryptic fig wasp species. mtDNA clade diversity declines from all four present in Northern Queensland to just one in Sydney, near the southern range limit. However, at most sites multiple clades coexist and can be found in the same tree or even the same fig fruit and there is no evidence for parallel sub-division of the host fig species. Both mtDNA data and sequences from two nuclear genes support the monophyly of the
Optimality, plasticity and selective regime in fig wasp sex ratios
Evolutionary ecology of figs and their associates: Recent progress and outstanding puzzles
The evolution of mutualisms: Exploring the paths between conflict and cooperation
How to be a dioecious fig: Chemical mimicry between sexes matters only when both sexes flower synchronously
DOI:10.1038/srep21236
URL
PMID:26888579
[本文引用: 1]

In nursery pollination mutualisms, which are usually obligate interactions, olfactory attraction of pollinators by floral volatile organic compounds (VOCs) is the main step in guaranteeing partner encounter. However, mechanisms ensuring the evolutionary stability of dioecious fig-pollinator mutualisms, in which female fig trees engage in pollination by deceit resulting in zero reproductive success of pollinators that visit them, are poorly understood. In dioecious figs, individuals of each sex should be selected to produce odours that their pollinating wasps cannot distinguish, especially since pollinators have usually only one choice of a nursery during their lifetime. To test the hypothesis of intersexual chemical mimicry, VOCs emitted by pollen-receptive figs of seven dioecious species were compared using headspace collection and gas chromatography-mass spectrometry analysis. First, fig-flower scents varied significantly among species, allowing host-species recognition. Second, in species in which male and female figs are synchronous, intersexual VOC variation was not significant. However, in species where figs of both sexes flower asynchronously, intersexual variation of VOCs was detectable. Finally, with one exception, there was no sexual dimorphism in scent quantity. We show that there are two ways to use scent to be a dioecious fig based on differences in flowering synchrony between the sexes.
Floral scents: Their roles in nursery pollination mutualisms
Progress on the breakdown of one-to-one rule in symbiosis of figs and their pollinating wasps
榕-传粉榕小蜂非一对一共生关系的研究进展
Fitness reduction for uncooperative fig wasps through reduced offspring size: A third component of host sanctions
DOI:10.1002/ecy.1471
URL
PMID:27859079
[本文引用: 4]

Mutually beneficial interactions between two species-mutualisms-are ancient, diverse, and of fundamental ecological importance. Nonetheless, factors that prevent one partner from reaping the benefits of the interaction without paying the cost are still poorly understood. Fig trees and their unique pollinators, fig wasps, present a powerful model system for studying mutualism stability. Both partners depend completely on each other for reproduction, cooperation levels can be manipulated, and the resulting field-based fitness quantified. Previous work has shown that fig trees can impose two types of host sanctions that reduce the fitness of wasps that do not pollinate: (1) fig abortion, which kills all developing larvae, and (2) reduced number of wasp offspring in figs that are not aborted. Here we demonstrate a third component of host sanctions. Through manipulative field experiments, we show that for four of five studied species, offspring of pollen-free foundresses are only 50-90% the size of offspring of pollinating foundresses. We further show that in all four studied species, smaller wasps are less likely to reach and enter a flowering fig to become foundresses themselves. Therefore, the experimentally determined size reduction of offspring is estimated to cause an additional reduction of up to 80% in fitness for a pollen-free foundress. We determine that the size reduction of pollen-free offspring acts on the level of the entire fig fruit rather than on individual flowers. These results show that estimates of the fitness effect of host sanctions on uncooperative symbionts should consider not only offspring quantity but also offspring quality. We discuss implications beyond the fig tree-fig wasp mutualism.
Host sanctions and pollinator cheating in the fig tree-fig wasp mutualism
Host sanctions in Panamanian Ficus are likely based on selective resource allocation
Precision of host sanctions in the fig tree-fig wasp mutualism: Consequences for uncooperative symbionts
DOI:10.1111/j.1461-0248.2012.01857.x
URL
PMID:22925044
[本文引用: 4]

Host sanctions that reduce the relative fitness of uncooperative symbionts provide a mechanism that can limit cheating and thus stabilise mutualisms over evolutionary timescales. Sanctions have been demonstrated empirically in several mutualisms. However, if multiple individual symbionts interact with each host, the precision with which individual cheating symbionts are targeted by host sanctions is critical to their short- and long-term effectiveness. No previous empirical study has directly addressed this issue. Here, we report the precision of host sanctions in the mutualism between fig trees and their pollinating wasps. Using field experiments and molecular parentage analyses, we show that sanctions in Ficus nymphaeifolia act at the level of entire figs (syconia), not at the level of the individual flowers within. Such fig-level sanctions allow uncooperative wasps, which do not bring pollen, to avoid sanctions in figs to which other wasps bring pollen. We discuss the relevance of sanction precision to other mutualisms.
Why mutualist partners vary in quality: Mutation-selection balance and incentives to cheat in the fig tree-fig wasp mutualism
Pollination mode in fig wasps: The predictive power of correlated traits
Selective resource allocation may promote a sex ratio in pollinator fig wasps more beneficial for the host tree
DOI:10.1038/srep35159
URL
PMID:27731351
[本文引用: 1]

Mutualisms play a key role in most ecosystems, yet the mechanisms that prevent overexploitation of the mutualistic relationship are still poorly understood. In the mutualism between fig trees and their pollinating wasps both partners depend on each other. Fig trees benefit from female wasps that disperse their pollen, whereas wasps frequently benefit from a higher ratio of male offspring. Here we use manipulative field experiments to address whether host trees (Ficus racemosa) can influence the offspring sex ratio of the pollinator wasp. We controlled wasp matings; virgin wasps can lay only male eggs. We found that virgin foundress wasps had fewer offspring than mated foundresses. This was not caused by virgin wasps having a shorter lifespan, or laying fewer eggs. Instead, male wasp larvae were more likely to die during development. Additionally, male eggs were deposited in flowers of equal style length to those of female eggs, yet emerged from galls with shorter pedicels than those of female wasps. We suggest that male larvae are either allocated less resources by the tree, or are less able to attract resources, during development. If the tree orchestrates this difference it would promote a more female-biased wasp brood, thus increasing the tree's fitness.
Critical review of host specificity and its coevolutionary implications in the fig/fig-wasp mutualism
Rapid evolution of pollinator-mediated plant reproductive isolation
Cryptic species of fig-pollinating wasps: Implications for the evolution of the fig-wasp mutualism, sex allocation, and precision of adaptation
Figs (Ficus spp.) and fig wasps (Chalcidoidea, Agaonidae): Hypotheses for an ancient symbiosis
Co-occurrence of two Eupristina species on Ficus altissima in Xishuangbanna, SW China
Signalling receptivity: Comparison of the emission of volatile compounds by figs of Ficus hispida before, during and after the phase of receptivity to pollinators
Comparative genomics reveals convergent rates of evolution in ant-plant mutualisms
On the rate of DNA sequence evolution in Drosophila
DOI:10.1007/BF02603075
URL
PMID:2501501
[本文引用: 1]

Analysis of the rate of nucleotide substitution at silent sites in Drosophila genes reveals three main points. First, the silent rate varies (by a factor of two) among nuclear genes; it is inversely related to the degree of codon usage bias, and so selection among synonymous codons appears to constrain the rate of silent substitution in some genes. Second, mitochondrial genes may have evolved only as fast as nuclear genes with weak codon usage bias (and two times faster than nuclear genes with high codon usage bias); this is quite different from the situation in mammals where mitochondrial genes evolve approximately 5-10 times faster than nuclear genes. Third, the absolute rate of substitution at silent sites in nuclear genes in Drosophila is about three times higher than the average silent rate in mammals.
Evidence for intersexual chemical mimicry in a dioecious plant
Faster speciation of fig-wasps than their host figs leads to decoupled speciation dynamics: Snapshots across the speciation continuum
DOI:10.1111/mec.15190
URL
PMID:31338917
[本文引用: 2]

Even though speciation involving multiple interacting partners, such as plants and their pollinators, has attracted much research, most studies focus on isolated phases of the process. This currently precludes an integrated understanding of the mechanisms leading to cospeciation. Here, we examine population genetic structure across six species-pairs of figs and their pollinating wasps along an elevational gradient in New Guinea. Specifically, we test three hypotheses on the genetic structure within the examined species-pairs and find that the hypothesized genetic structures represent different phases of a single continuum, from incipient cospeciation to the full formation of new species. Our results also illuminate the mechanisms governing cospeciation, namely that fig wasps tend to accumulate population genetic differences faster than their figs, which initially decouples the speciation dynamics between the two interacting partners and breaks down their one-to-one matching. This intermediate phase is followed by genetic divergence of both partners, which may eventually restore the one-to-one matching among the fully formed species. Together, these findings integrate current knowledge on the mechanisms operating during different phases of the cospeciation process. They also reveal that the increasingly reported breakdowns in one-to-one matching may be an inherent part of the cospeciation process. Mechanistic understanding of this process is needed to explain how the extraordinary diversity of species, especially in the tropics, has emerged. Knowing which breakdowns in species interactions are a natural phase of cospeciation and which may endanger further generation of diversity seems critical in a constantly changing world.
Parasite zoonoses and wildlife: One health, spillover and human activity
DOI:10.1016/j.ijpara.2013.06.007
URL
PMID:23892130
[本文引用: 1]

This review examines parasite zoonoses and wildlife in the context of the One Health triad that encompasses humans, domestic animals, wildlife and the changing ecosystems in which they live. Human (anthropogenic) activities influence the flow of all parasite infections within the One Health triad and the nature and impact of resulting spillover events are examined. Examples of spillover from wildlife to humans and/or domestic animals, and vice versa, are discussed, as well as emerging issues, particularly the need for parasite surveillance of wildlife populations. Emphasis is given to Trypanosoma cruzi and related species in Australian wildlife, Trichinella, Echinococcus, Giardia, Baylisascaris, Toxoplasma and Leishmania.
A trophic cascade induced by predatory ants in a fig-fig wasp mutualism
Discriminative host sanctions in a fig-wasp mutualism
DOI:10.1890/13-0749.1
URL
PMID:25000769
[本文引用: 1]

In some mutualisms, cooperation in symbionts is promoted by hosts sanctioning
Interference competition and high temperatures reduce the virulence of fig wasps and stabilize a fig-wasp mutualism
Discriminative host sanction together with relatedness promote the cooperation in fig-fig wasp mutualism
Asymmetric interaction and indeterminate fitness correlation between cooperative partners in the fig-fig wasp mutualism
Spatial heterogeneity and host repression in fig-fig wasp mutualism
DOI:10.1007/s11427-015-4848-x
URL
PMID:25863497
[本文引用: 1]

It is generally believed that physical heterogeneity in common resource or evolutionary restraint can sufficiently prevent direct conflict between host and symbionts in mutualism systems. Our data on fig/fig wasp reciprocal mutualism (Ficus racemosa), however, show that structural barriers of female flowers or genetic constraints of pollinators previously hypothesized exist, but cannot sufficiently maintain the mutualism stability. The results show that a positive relationship between seed and wasp production could be maintained in warm season, which might be because of density dependence restraint among foundresses and their low oviposition and pollination efficiency, keeping common resource (female flowers) utilization unsaturated. Whilst, a negative correlation between wasp offspring and viable seed production was also observed in cold season, which might be that the increased oviposition and pollination efficiency maximized the common resource utilization. The fitness trade-off between fig and pollinator wasps is greatly affected by environmental or ecological variations. The local stability might result from temporal low exploitation efficiency of pollinators together with interference competition among pollinators. We suggest that host repression through the active regulation of bract closure, which can create interference competition among the foundresses and prevent extra more foundresses sequential entry in fruit cavities, would help the figs avoiding the cost of over-exploitation. This essentially takes the same role as sanctioning of cheating or competitive behaviors.
How to be a fig wasp
DOI:10.1146/annurev.ento.47.091201.145213
URL
PMID:11729077
[本文引用: 8]

In the two decades since Janzen described how to be a fig, more than 200 papers have appeared on fig wasps (Agaonidae) and their host plants (Ficus spp., Moraceae). Fig pollination is now widely regarded as a model system for the study of coevolved mutualism, and earlier reviews have focused on the evolution of resource conflicts between pollinating fig wasps, their hosts, and their parasites. Fig wasps have also been a focus of research on sex ratio evolution, the evolution of virulence, coevolution, population genetics, host-parasitoid interactions, community ecology, historical biogeography, and conservation biology. This new synthesis of fig wasp research attempts to integrate recent contributions with the older literature and to promote research on diverse topics ranging from behavioral ecology to molecular evolution.
Correlated evolution in fig pollination
Reproductive characteristics of pollinator and cheater wasps that utilize the female flowers of Ficus altissima
高榕雌花期传粉榕小蜂和欺骗性小蜂的繁殖特点
Species composition and diversity of fig wasps and figs in Yunnan
云南省榕小蜂和榕树的物种组成及多样性
The incidence and pattern of copollinator diversification in dioecious and monoecious figs
DOI:10.1111/evo.12584
URL
PMID:25495152
[本文引用: 2]

Differences in breeding system are associated with correlated ecological and morphological changes in plants. In Ficus, dioecy and monoecy are strongly associated with different suites of traits (tree height, population density, fruiting frequency, pollinator dispersal ecology). Although approximately 30% of fig species are pollinated by multiple species of fig-pollinating wasps, it has been suggested that copollinators are rare in dioecious figs. Here, we test whether there is a connection between the fig breeding system and copollinator incidence and diversification by conducting a meta-analysis of molecular data from pollinators of 119 fig species that includes new data from 15 Asian fig species. We find that the incidence of copollinators is not significantly different between monoecious and dioecious Ficus. Surprisingly, while all copollinators in dioecious figs are sister taxa, only 32.1% in monoecious figs are sister taxa. We present hypotheses to explain those patterns and discuss their consequences on the evolution of this mutualism.
Oviposition strategies, host coercion and the stable exploitation of figs by wasps
Coexistence of cryptic species
Non-pollinating cheater wasps benefit from seasonally poor performance of the mutualistic pollinating wasps at the northern limit of the range of Ficus microcarpa
Differential deployment of sanctioning mechanisms by male and female host trees in a gynodioecious fig-wasp mutualism
DOI:10.1002/ecy.2597
URL
PMID:30615203

In some insect nursery pollination mutualisms, plant hosts impose net costs to uncooperative
Effects of foundress number, foundresses entry interval and non-pollinating wasps on clutch size and offspring sex ratio of pollinating fig wasps (Hymenoptera: Agaonidae)
母代雌蜂数、进果时间及非传粉小蜂对传粉榕小蜂后代数量及性比的影响