

中甸乌头总状花序不同位置花粉和花蜜的化学性状没有显著差异
收稿日期: 2023-10-04
录用日期: 2023-12-04
网络出版日期: 2023-12-09
基金资助
国家自然科学基金(32360262);国家自然科学基金(31901208);国家自然科学基金委员会贵州省人民政府喀斯特科学研究中心项目(U1812401)
No significant differences found in chemical traits of pollen and nectar located in different positions across Aconitum piepunense racemes
Received date: 2023-10-04
Accepted date: 2023-12-04
Online published: 2023-12-09
植物花序中的资源配置通常在花发育的时间或者位置上存在差异。植物的化学性状在自身生长发育以及植物与环境的相互作用中发挥重要作用, 但是化学性状是否存在花序内的差异, 目前还不清楚。为了解花序中不同位置上花报酬(花粉和花蜜)化学性状的差异, 本文以总状花序的中甸乌头(Aconitum piepunense)为研究对象, 从下往上, 将花序中不同位置的花分为基部花、中部花和顶部花, 观察传粉者在花序中的访问特点, 测量不同位置花的花蜜体积和糖浓度, 分别检测分析不同位置花的花粉和花蜜的化学物质的种类和相对含量。结果表明: 中甸乌头的传粉者弗里熊蜂(Bombus friseanus)和圣熊蜂(B. religiosus)通常由基部到顶部进行访花, 对不同位置花的单花访问时间和访花频率不存在显著性差异。基部花的花蜜体积高于中部花、顶部花, 3个位置的花蜜糖浓度不存在显著差异。花粉和花蜜中次级代谢物的相对含量和种类数都显著高于初级代谢物的相对含量和种类, 花粉中初级代谢物和次级代谢物的相对含量和种类数都显著高于花蜜。花粉和花蜜中的绝大部分的化学物质的相对含量和种类数在花序中的3个位置整体上不存在显著性差异。研究表明熊蜂为主要传粉者的中甸乌头, 其总状花序内上、中、下3个位置花的花粉和花蜜的化学性状不存在明显的结构效应。
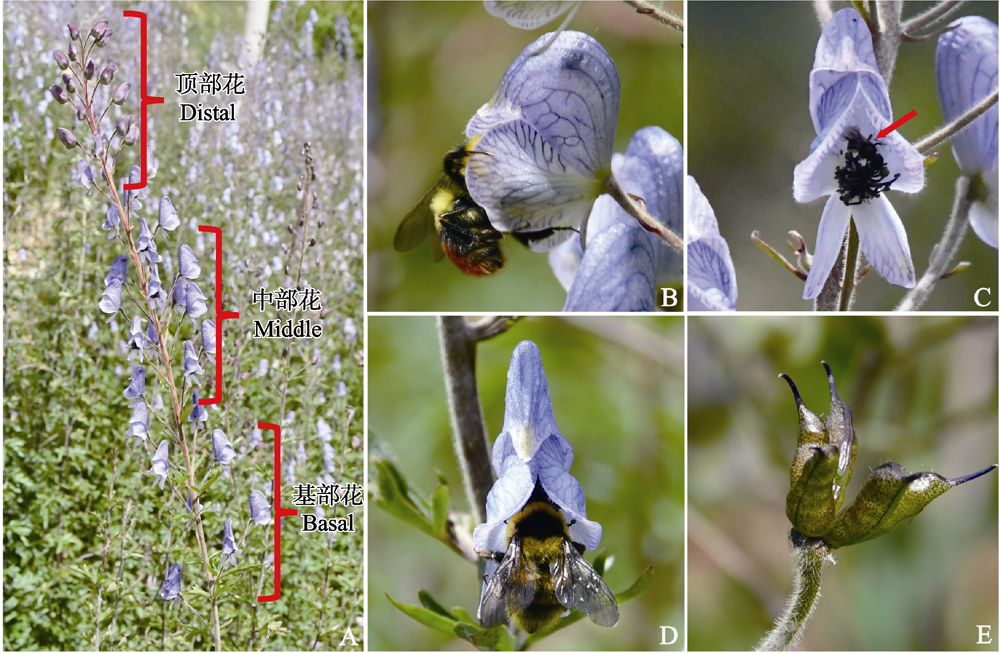
吕晓琴 , 李杨 , 王顺雨 , 姚仁秀 , 王晓月 . 中甸乌头总状花序不同位置花粉和花蜜的化学性状没有显著差异[J]. 生物多样性, 2024 , 32(1) : 23371 . DOI: 10.17520/biods.2023371
Aims: Floral resource allocation often varies across developmental time and across locations in inflorescences. The chemical profile of a plant plays an important role in its growth, development, and interaction with its environment; however, it remains unclear whether such chemical traits vary among floral positions in a single plant. We aim to investigate the variation in chemical profiles of floral reward (pollen and nectar) at different positions of a single plant’s inflorescences.
Methods: Flowers in racemes of Aconitum piepunense (aconite) were categorized into three positions (basal, middle and distal). In each of the three positions, we observed pollinator foraging behaviors and measured nectar volume and sugar concentration. We then measured and analyzed the types and relative contents of chemical compounds within the pollen and nectar of flowers at each of the three positions.
Results: The two major pollinators (Bombus friseanus and B. religiosus) typically visited flowers from basal to distal in a sequential order in A. piepunense. Neither the bumblebee visit frequency nor the duration per flower significantly differed among the three positions. Flowers at the bottom of each position secreted more nectar by volume than the middle and upper flowers, but this volume was not significantly different in terms of the nectar sugar concentration across positions. We also found that the relative contents and types of secondary metabolites within the pollen and nectar were both significantly higher than those of primary metabolites. Further, the relative contents and classes of secondary metabolites were significantly higher in pollen than in nectar. Finally, we observed that the relative content and classes of most chemical profiles within pollen or nectar did not significantly differ at basal, middle, or distal flower positions.
Conclusion: Our results indicate that in the raceme of bumblebee-pollinated A. piepunense, floral positioning within a single plant has no clear impact on the chemical profiles of its pollen and nectar.

Key words: Aconitum piepunense; visiting behavior; pollen; nectar; chemical character; raceme; architectural effect
| [1] | Adler LS (2000) The ecological significance of toxic nectar. Oikos, 91, 409-420. |
| [2] | Adler LS, Irwin RE (2012) Nectar alkaloids decrease pollination and female reproduction in a native plant. Oecologia, 168, 1033-1041. |
| [3] | Arnold SEJ, Idrovo MEP, Arias LJL, Belmain SR, Stevenson PC (2014) Herbivore defence compounds occur in pollen and reduce bumblebee colony fitness. Journal of Chemical Ecology, 40, 878-881. |
| [4] | Ashman TL, Hitchens MS (2000) Dissecting the causes of variation in intra-inflorescence allocation in a sexually polymorphic species, Fragaria virginiana (Rosaceae). American Journal of Botany, 87, 197-204. |
| [5] | Baker HG (1977) Non-sugar chemical constituents of nectar. Apidologie, 8, 349-356. |
| [6] | Barlow SE, Wright GA, Ma C, Barberis M, Farrell IW, Marr EC, Brankin A, Pavlik BM, Stevenson PC (2017) Distasteful nectar deters floral robbery. Current Biology, 27, 2552-2558. |
| [7] | Brochu KK, van Dyke MT, Milano NJ, Petersen JD, McArt SH, Nault BA, Kessler A, Danforth BN (2020) Pollen defenses negatively impact foraging and fitness in a generalist bee (Bombus impatiens: Apidae). Scientific Reports, 10, 3112-3112. |
| [8] | Brunet J, Charlesworth D (1995) Floral sex allocation in sequentially blooming plants. Evolution, 49, 70-79. |
| [9] | Buchanan BB, Gruissem W, Jones RL (translated by Qu LJ, Gu HY, Bai SN, Zhao JD, Chen ZL (2003) Biochemistry & Molecular Biology of Plants, pp. 1026-1082. Science Press, Beijing. (in Chinese) |
| [瞿礼嘉, 顾红雅, 白书农, 赵进东, 陈章良 译 (2003) 植物生物化学与分子生物学. 科学出版社, 北京.] | |
| [10] | Cane JH, Gardner DR, Weber M (2020) Neurotoxic alkaloid in pollen and nectar excludes generalist bees from foraging at death-camas, Toxicoscordion paniculatum (Melanthiaceae). Biological Journal of the Linnean Society, 131, 927-935. |
| [11] | Carlson JE, Harms KE (2006) The evolution of gender-biased nectar production in hermaphroditic plants. The Botanical Review, 72, 179-205. |
| [12] | Cook D, Manson JS, Gardner DR, Welch KD, Irwin RE (2013) Norditerpene alkaloid concentrations in tissues and floral rewards of larkspurs and impacts on pollinators. Biochemical Systematics and Ecology, 48, 123-131. |
| [13] | De-Melo AAM, de Almeida-Muradian LB (2017) Chemical composition of bee pollen. In: Bee Products—Chemical and Biological Properties (ed. Alvarez-Suarez JM), pp. 221-259. Springer, Cham (Switzerland). |
| [14] | Diggle PK (1995) Architectural effects and the interpretation of patterns of fruit and seed development. Annual Review of Ecology, Evolution, and Systematics, 26, 531-552. |
| [15] | Dübecke A, Beckh G, Lüllmann C (2011) Pyrrolizidine alkaloids in honey and bee pollen. Food Additives and Contaminants: Part A, 28, 348-358. |
| [16] | Erb M, Kliebenstein DJ (2020) Plant secondary metabolites as defenses, regulators, and primary metabolites: The blurred functional trichotomy. Plant Physiology, 184, 39-52. |
| [17] | Fisogni A, Cristofolini G, Rossi M, Galloni M (2011) Pollinator directionality as a response to nectar gradient: Promoting outcrossing while avoiding geitonogamy. Plant Biology, 13, 848-856. |
| [18] | Flamini G, Cioni PL, Morelli I (2003) Use of solid-phase micro-extraction as a sampling technique in the determination of volatiles emitted by flowers, isolated flower parts and pollen. Journal of Chromatography A, 998, 229-233. |
| [19] | Ge XYM, Lu HS, Tian H, Wu Y, Zhang DY, Liao WJ (2022) Male-biased sex allocation in late-blooming flowers driven by resource limitation in the clonal perennial Aconitum kusnezoffii (Ranunculaceae). Journal of Systematics and Evolution, 60, 1393-1404. |
| [20] | Gosselin M, Michez D, Vanderplanck M, Roelants D, Glauser G, Rasmont P (2013) Does Aconitum septentrionale chemically protect floral rewards to the advantage of specialist bumblebees? Ecological Entomology, 38, 400-407. |
| [21] | Heiling JM, Cook D, Lee ST, Irwin RE (2019) Pollen and vegetative secondary chemistry of three pollen-rewarding lupines. American Journal of Botany, 106, 643-655. |
| [22] | Huang SQ, Tang LL, Yu Q, Guo YH (2004) Temporal floral sex allocation in protogynous Aquilegia yabeana contrasts with protandrous species: Support for the mating environment hypothesis. Evolution, 58, 1131-1134. |
| [23] | Jacquemart AL, Buyens C, Hérent MF, Quetin-Leclercq J, Lognay G, Hance T, Quinet M (2019) Male flowers of Aconitum compensate for toxic pollen with increased floral signals and rewards for pollinators. Scientific Reports, 9, 16498. |
| [24] | Liu CQ, Huang SQ (2012) Does the relative importance of resource competition and architectural effect in floral variation vary with stages of floral ontogeny? Journal of Systematics and Evolution, 50, 119-124. |
| [25] | Mazer SJ, Dawson KA (2001) Size-dependent sex allocation within flowers of the annual herb Clarkia unguiculata (Onagraceae): Ontogenetic and among-plant variation. American Journal of Botany, 88, 819-831. |
| [26] | Nicolson SW, Nepi M, Pacini E (2007) Nectaries and nectar. In: Nectar Chemistry (eds Nicolson SW, Thornburg RW), pp. 215-264. Springer, Dordrecht. |
| [27] | Palmer-Young EC, Farrell IW, Adler LS, Milano NJ, Stevenson PC (2019) Chemistry of floral rewards: Intra- and interspecific variability of nectar and pollen secondary metabolites across taxa. Ecological Monographs, 89, e01335. |
| [28] | Pyke GH (1978) Optimal foraging: Movement patterns of bumblebees between inflorescences. Theoretical Population Biology, 13, 72-98. |
| [29] | Ritmejeryt? E, Boughton BA, Bayly MJ, Miller RE (2020) Unique and highly specific cyanogenic glycoside localization in stigmatic cells and pollen in the genus Lomatia (Proteaceae). Annals of Botany, 126, 387-400. |
| [30] | Sharma A, Sharma S, Kumar A, Kumar V, Sharma AK (2022) Plant secondary metabolites:An introduction of their chemistry and biological significance with physicochemical aspect. In: Plant Secondary Metabolites (eds Sharma AK, Sharma A), pp. 1-45. Springer, Singapore. |
| [31] | Solomon BP (1985) Environmentally influenced changes in sex expression in an andromonoecious plant. Ecology, 66, 1321-1332. |
| [32] | Solomon BP (1988) Patterns of pre- and postfertilization resource allocation within an inflorescence: Evidence for interovary competition. American Journal of Botany, 75, 1074-1079. |
| [33] | Stevenson PC (2020) For antagonists and mutualists: The paradox of insect toxic secondary metabolites in nectar and pollen. Phytochemistry Reviews, 19, 603-614. |
| [34] | Stevenson PC, Nicolson SW, Wright GA (2017) Plant secondary metabolites in nectar: Impacts on pollinators and ecological functions. Functional Ecology, 31, 65-75. |
| [35] | Thorp RW (2000) The collection of pollen by bees. Plant Systematics and Evolution, 222, 211-223. |
| [36] | Wang H, Zhang ZQ, Zhang B, Wang LP, Guo W, Fang Y, Li QJ (2022) Architectural effects regulate resource allocation within the inflorescences with nonlinear blooming patterns. American Journal of Botany, 109, 1191-1202. |
| [37] | Wang XY, Tang J, Wu T, Wu D, Huang SQ (2019) Bumblebee rejection of toxic pollen facilitates pollen transfer. Current Biology, 29, 1401-1406. |
| [38] | Wright GA, Baker DD, Palmer MJ, Stabler D, Mustard JA, Power EF, Borland AM, Stevenson PC (2013) Caffeine in floral nectar enhances a pollinator’s memory of reward. Science, 339, 1202-1204. |
| [39] | Yan XF, Wang Y, Li YM (2007) Plant secondary metabolism and its response to environment. Acta Ecologica Sinica, 27, 2554-2562. (in Chinese with English abstract) |
| [阎秀峰, 王洋, 李一蒙 (2007) 植物次生代谢及其与环境的关系. 生态学报, 27, 2554-2562.] | |
| [40] | Zangerl AR, Rutledge CE (1996) The probability of attack and patterns of constitutive and induced defense: A test of optimal defense theory. The American Naturalist, 147, 599-608. |
| [41] | Zhao ZG, Du GZ, Huang SQ (2010) The effect of flower position on variation and covariation in floral traits in a wild hermaphrodite plant. BMC Plant Biology, 10, 91. |
| [42] | Zhao ZG, Lu NN, Conner JK (2016) Adaptive pattern of nectar volume within inflorescences: Bumblebee foraging behavior and pollinator-mediated natural selection. Scientific Reports, 6, 34499. |
/
| 〈 |
|
〉 |