



Biodiv Sci ›› 2018, Vol. 26 ›› Issue (9): 988-997. DOI: 10.17520/biods.2018127 cstr: 32101.14.biods.2018127
• Reviews • Previous Articles Next Articles
Jinxiu Ke, Duo Chen, Yanping Guo*( )
)
Received:2018-04-26
Accepted:2018-06-17
Online:2018-09-20
Published:2019-01-05
Contact:
Guo Yanping
About author:# Co-first authors
Jinxiu Ke, Duo Chen, Yanping Guo. Designing leaf marginal shapes: Regulatory mechanisms of leaf serration or dissection[J]. Biodiv Sci, 2018, 26(9): 988-997.
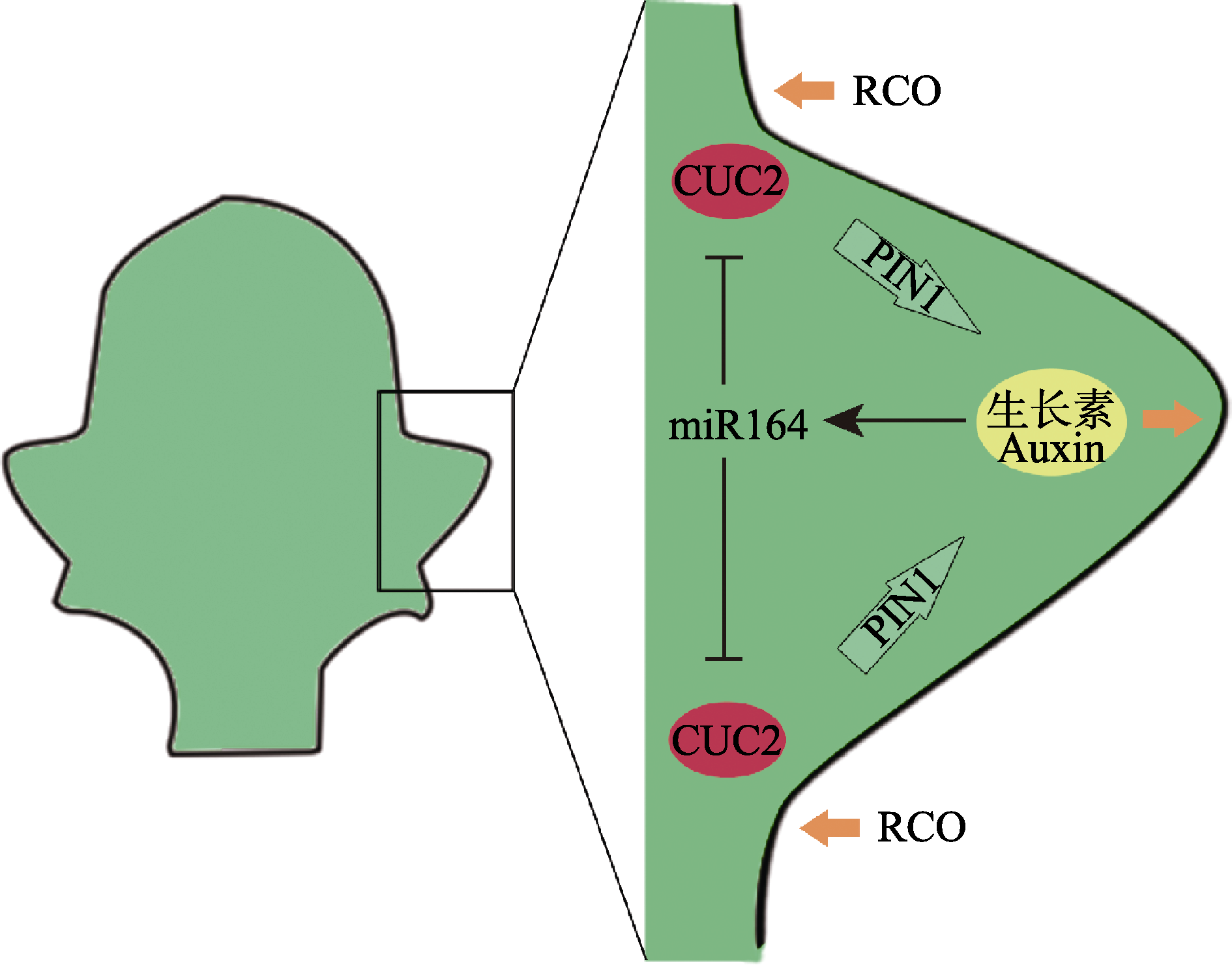
Fig. 1 A model for the molecular regulation of the development of leaf marginal serration according to studies on Arabidopsis thaliana and Cardamine hirsute (Barkoulas et al, 2007; Runions & Tsiantis, 2017). At the heart of the model is a feedback loop between CUC2 and auxin activities. CUC2 is required for PIN1-mediated auxin polar transport (horrow arrows); in turn, auxin activity maxima at the tip of the developing serration activates miR164 which represses CUC2 posttranscriptionally and generates an interspersed pattern of auxin maxima and CUC2 expression at the leaf margin. MIR164 and CUC2 are expressed in partially overlapping regions at the sinus of the serrations. Auxin enhances outgrowth of the serrations. There are additional growth regulators modulating leaf growth to shape the form of protrusions, for instance, RCO inhibits growth in indentations, producing more dissected forms.
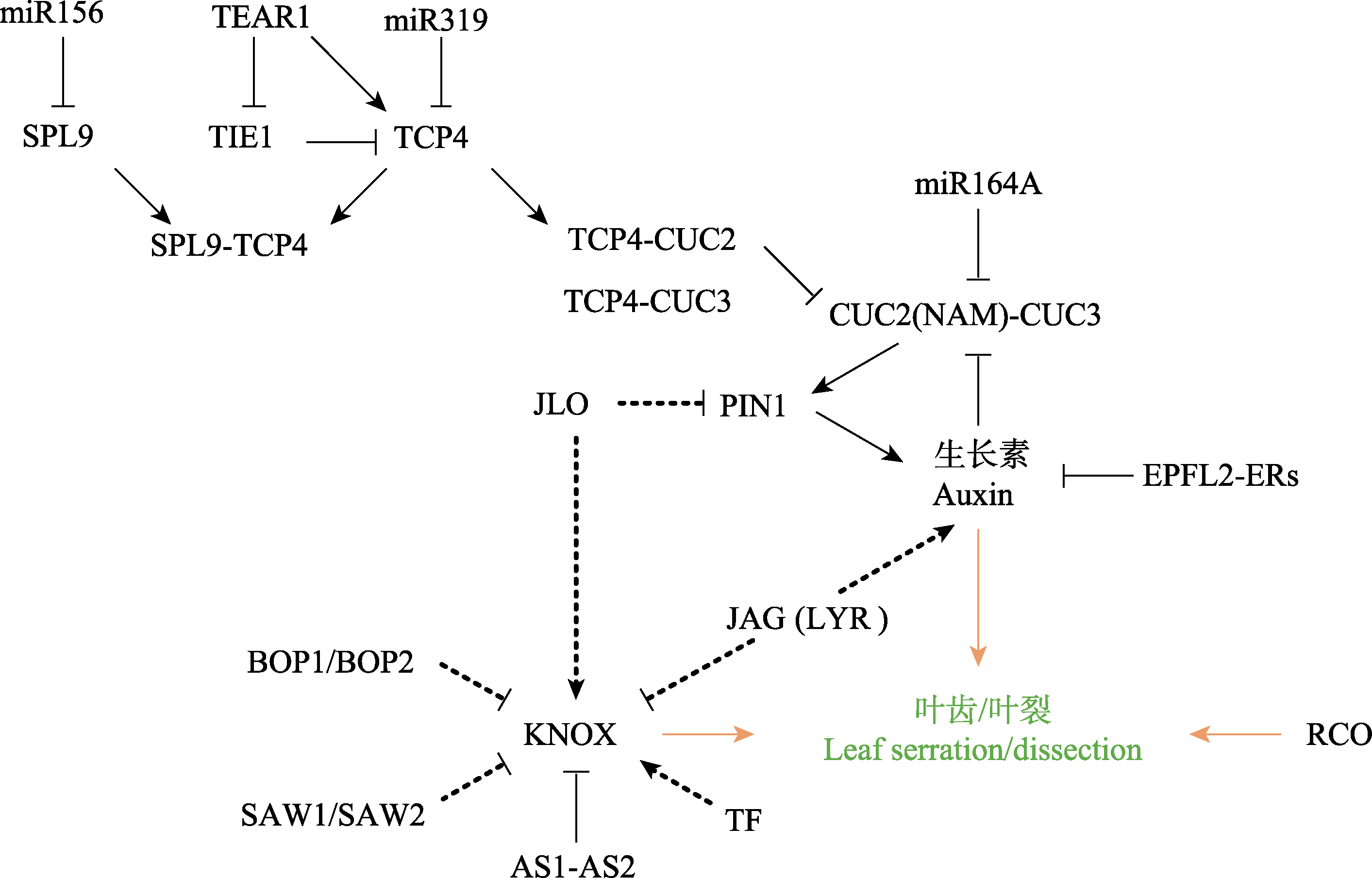
Fig. 2 The molecular regulating network underlining the development leaf serration/dissection. The solid lines indicate the relatively clear interactions between molecules, while the dotted lines suggest unsure interactions reported by a few studies. The orange arrows suggest promotion of leaf serration/dissection.
| [1] | Aida M, Ishida T, Fukaki H, Fujisawa H, Tasaka M (1997) Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. The Plant Cell, 9, 841-857. |
| [2] | Bar M, Ori N (2014) Leaf development and morphogenesis. Development, 141, 4219-4230. |
| [3] | Bar M, Ori N (2015) Compound leaf development in model plant species. Current Opinion in Plant Biology, 23, 61-69. |
| [4] | Barkoulas M, Galinha C, Grigg SP, Tsiantis M (2007) From genes to shape: Regulatory interactions in leaf development. Current Opinion in Plant Biology, 10, 660-666. |
| [5] | Barkoulas M, Hay A, Kougioumoutzi E, Tsiantis M (2008) A developmental framework for dissected leaf formation in the Arabidopsis relative Cardamine hirsuta. Nature Genetics, 40, 1136-1141. |
| [6] | Benkova E, Michniewicz M, Sauer M, Teichmann T, Seifertova D, Jurgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell, 115, 591-602. |
| [7] | Berger Y, Harpaz-Saad S, Brand A, Melnik H, Sirding N, Alvarez JP, Zinder M, Samach A, Eshed Y, Ori N (2009) The NAC-domain transcription factor GOBLET specifies leaflet boundaries in compound tomato leaves. Development, 136, 823-832. |
| [8] | Bharathan G, Goliber TE, Moore C, Kessler S, Pham T, Sinha NR (2002) Homologies in leaf form inferred from KNOX gene expression during development. Science, 296, 1858-1860. |
| [9] | Bilsborough GD, Runions A, Barkoulas M, Jenkins HW, Hasson A, Galinha C, Laufs P, Hay A, Prusinkiewicz P, Tsiantis M (2011) Model for the regulation of Arabidopsis thaliana leaf margin development. Proceedings of the National Academy of Sciences, USA, 108, 3424-3429. |
| [10] | Blein T, Hasson A, Laufs P (2010) Leaf development: What it needs to be complex. Current Opinion in Plant Biology, 13, 75-82. |
| [11] | Blein T, Pulido A, Vialette-Guiraud A, Nikovics K, Morin H, Hay A, Johansen IE, Tsiantis M, Laufs P (2008) A conserved molecular framework for compound leaf development. Science, 322, 1835-1839. |
| [12] | Bonn S, Furlong E (2008) cis-Regulatory networks during development: A view of Drosophila. Current Opinion in Genetics and Development, 18, 513-520. |
| [13] | Borghi L, Bureau M, Simon R (2007) Arabidopsis JAGGED LATERAL ORGANS is expressed in boundaries and coordinates KNOX and PIN activity. The Plant Cell, 19, 1795-1808. |
| [14] | Byrne ME (2005) Shoot development-genetic interactions in the meristem. Biochemical Society Transactions, 33, 1499-1501. |
| [15] | Byrne ME, Simorowski J, Martienssen RA (2002) ASYMMETRIC LEAVES1 reveals KNOX gene redundancy in Arabidpsis. Development, 129, 1957-1965. |
| [16] | Carroll S (2008) Evo-devo and an expanding evolutionary synthesis: A genetic theory of morphological evolution. Cell, 134, 25-36. |
| [17] | Cheng X, Peng J, Ma J, Tang Y, Chen R, Mysore KS, Wen J (2012) NO APICAL MERISTEM (MtNAM) regulates floral organ identityand lateral organ separation in Medicago truncatula. New Phytologist, 195, 71-84. |
| [18] | Chitwood DH, Sinha NR (2014) Plant development: Small RNAs and the metamorphosis of leaves. Current Biology, 24, R1087-R1089. |
| [19] | Chitwood DH, Sinha NR (2016) Evolutionary and environmental forces sculpting leaf development. Current Biology, 26, R297-R306. |
| [20] | Chitwood DH, Sinha NR (2004) Small RNAs and the metamorphosis of leaves. Current Biology, 22, 1087-1089. |
| [21] | David-Schwartz R, Koenig D, Sinha NR (2009) LYRATE is a key regulator of leaflet initiation and lamina outgrowth in tomato. The Plant Cell, 21, 3093-3104. |
| [22] | Dengler NG, Tsukaya H (2001) Leaf morphogenesis in dicotyledons: Current issues. International Journal of Plant Sciences, 162, 459-464. |
| [23] | Dinneny JR, Yadegari R, Fiseher RL, Yanofsky MF, Weigel D (2004) The role of JAGGED in shaping lateral organs. Development, 131, 1101-1110. |
| [24] | Efroni I, Eshed Y, Lifschitz E (2010) Morphogenesis of simple and compound leaves: A critical review. The Plant Cell, 22, 1019-1032. |
| [25] | Ha CM, Jun JH, Fleteher JC (2010) Control of Arabidopsis leaf morphogenesis through regulation of the YABBY and KNOX families of transcription factors. Genetics, 186, 197-206. |
| [26] | Ha CM, Kim GT, Kim BC, Jun JH, Soh MS, Ueno Y, Maehida Y, Tsukaya H, Nam HG (2003) The BLADE-ON-PETIOLE 1 gene controls leaf pattern formation through the modulation of meristematic activity in Arabidopsis. Development, 130, 161-172. |
| [27] | Hake S, Smith HMS, Holtan H, Magnani E, Mele G, Ramirez J (2004) The role of KNOX genes in plant development. Annual Review of Cell Developmental Biology, 20, 125-151. |
| [28] | Hareven D, Gutfinger T, Parnis A, Eshed Y, Lifschitz E (1996) The making of a compound leaf: Genetic manipulation of leaf architecture in tomato. Cell, 84, 735-744. |
| [29] | Hasson A, Plessis A, Blein T, Adroher B, Grigg S, Tsiantis M, Boudaoud A, Damerval C, Laufs P (2011) Evolution and diverse roles of the CUP-SHAPEDCOTYLEDON genes in Arabidopsis leaf development. The Plant Cell, 23, 54-68. |
| [30] | Hay A, Tsiantis M (2006) The genetic basis for differences in leaf form between Arabidopsis thaliana and its wild relative Cardamine hirsuta. Nature Genetics, 38, 942-947. |
| [31] | Hay A, Barkoulas M, Tsiantis M (2006) ASYMMETRIC LEAVES1 and auxin activities converge to repress BREVIPEDICELLUS expression and promote leaf development in Arabidopsis. Development, 133, 3955-3961. |
| [32] | Hay A, Tsiantis M (2010) KNOX genes: Versatile regulators of plant development and diversity. Development, 137, 3153-3165. |
| [33] | Heisler MG, Jonsson H (2006) Modeling auxin transport and plant development. Journal of Plant Growth Regulation, 25, 302-312. |
| [34] | Hui MX, Zhang LG, Zhang MK (2012) Research progress on the molecular mechanism of leaf margins. In: Proceedings of the 14th Symposium of the Crucifers Subdivision of the Chinese Society for Horticultural Science, pp. 52-59. (in Chinese) |
| [惠麦霞, 张鲁刚, 张明科 (2012) 植物叶缘发育分子机理研究进展. 见:中国园艺学会十字花科分会第十四届学术研讨会论文集, 52-59页.] | |
| [35] | Jun JH, Ha CM, Fletcher JC (2010) BLADE-ON-PETIOLE1 coordinates organ determinacy and axial polarity in Arabidopsis by directly activating ASYMMETRIC LEAVES2. Plant Cell, 22, 62-76. |
| [36] | Kasprzewska A, Carter R, Swarup R, Bennett M, Monk N, Hobbs JK, Fleming A (2015) Auxin influx importers modulate serration along the leaf margin. The Plant Journal, 83, 705-718. |
| [37] | Kawamura E, Horiguchi G, Tsukaya H (2010) Mechanisms of leaf tooth formation in Arabidopsis. The Plant Journal, 62, 429-441. |
| [38] | Kidner CA, Umbreen SU (2010) Why is leaf shape so variable. International Joumal of Plant Developmental Biology, 4(Special Issue 1), 64-75. |
| [39] | Koyama T, Sato F, Ohme-Takagi M (2017) Roles of miR319 and TCP transcription factors in leaf development. Plant Physiology, 175, 874-885. |
| [40] | Kramer EM (2004) PIN and AUX/LAX proteins: Their role in auxin accumulation. Trends in Plant Science, 9, 578-582. |
| [41] | Kumar R, Kushalappa K, Godt D, Pidkowich MS, Pastorelli S, HePworth SR, Haughn GW (2007) The Arabidopsis BEL1-LIKE HOMEODOMAIN proteins SAW1 and SAW2 act redundantly to regulate KNOX expression spatially in leaf margins. The Plant Cell, 19, 2719-2735. |
| [42] | Ledford H (2018) The lost art of looking at plants. Nature, 553, 396-398. |
| [43] | Moon J, Hake S (2011) How a leaf gets its shape. Current Opinion in Plant Biology, 14, 24-30. |
| [44] | Naz AA, Raman S, Martinez CC, Sinha NR, Schmitz G, Theres K (2013) Trifoliate encodes an MYB transcription factor that modulates leaf and shoot architecture in tomato. Proceedings of the National Academy of Sciences, USA, 110, 2401-2406. |
| [45] | Nikovics K, Blein T, Peaucelle A, Ishida T, Morin H, Aida M, Laufs P (2006) The balance between the MIR164A and CUC2 genes controls leaf margin serration in Arabidopsis. The Plant Cell, 18, 2929-2945. |
| [46] | Norberg M, Holmlund M, Nilsson O (2005) The BLADE ON PETIOLE genes act redundantly to control the growth and development of lateral organs. Development, 132, 2203-2213. |
| [47] | Ostria GE, Ranjan A, Zumstein K, Chitwood DH, Kumar R, Townsley BT, Ichihashi Y, Corcuera LJ, Sinha NR (2016) Transcriptomic analysis suggests a key role for SQUAMOSA PROMOTER BINDING PROTEIN LIKE, NAC and YUCCA genes in the heteroblastic development of the temperate rainforest tree Gevuina avellana (Proteaceae). New Phytologist, 210, 694-708. |
| [48] | Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature, 425, 257-263. |
| [49] | Petrasek J, Mravec J, Bouchard R, Blakeslee JJ, Abas M, Seifertova D, Niewska JW, Tadele Z, Kubes M, Covanova M, Dhonukshe P, Skupa P, Benkova E, Perry L, Krecek P, Lee OR, Fink GR, Geisler M, Murphy AS, Luschnig C, Zazimalova E, Frim J (2006) PIN proteins perform a rate-limiting function in cellular auxin efflux. Science, 312, 914-918. |
| [50] | Piazza P, Bailey CD, Cartolano M, Krieger J, Cao J, Ossowski S, Sehneeberger K, He F, de Meaux J, Hall N, Maeleod N, Filatov D, Hay A, Tsiantis M (2010) Arabidopsis thaliana leaf form evolved via loss of KNOX expression in leaves in association with a selective sweep. Current Biology, 20, 2223-2228. |
| [51] | Rubio-Somoza I, Zhou CM, Confraria A, Martinho C, von Born P, Baena-Gonzalez E, Wang JW, Weigel D (2014) Temporal control of leaf complexity by miRNA-regulated licensing of protein complexes. Current Biology, 24, 2714-2719. |
| [52] | Runions A, Tsiantis M (2017) The shape of things to come: From typology to predictive models for leaf diversity. American Journal of Botany, 104, 1437-1441. |
| [53] | Sha S, Chen D, Liu M, Li KL, Jiang CK, Wang DH, Guo YP (2018) To be serrate or pinnate: Diverse leaf forms of yarrows (Achillea) are linked to differential expression patterns of NAM genes. Annals of Botany, 121, 255-266 |
| [54] | Shani E, Burko Y, Ben-Yaakov L, Berger Y, Amsellem Z, Goldshmidt A, Sharon E, Ori N (2009) Stage-specific regulation of Solanum lycopersicum leaf maturation by Class KNOTTED1-LIKE HOMEOBOX proteins. The Plant Cell, 21, 3078-3092. |
| [55] | Siso S, Camarero JJ, Gil-Pelegrin E (2001) Relationship between hydraulic resistance and leaf morphology in broad leaf Quercus species: A new interpretation of leaf lobation. Trees, 15, 341-345. |
| [56] | Souer E, van Houwelingen A, Kloos D, Mol J, Koeset R (1996) The No Apical Meristem gene of petunia is required for pattern formation in embryos and flowers and is expressed at meristem and primordia boundaries. Cell, 85, 159-170. |
| [57] | Tameshige T, Okamoto S, Lee JS, Aida M, Tasaka M, Torii KU, Uchida N (2016) A secreted peptide and its receptors shape the auxin response pattern and leaf margin morphogenesis. Current Biology, 26, 2478-2485. |
| [58] | Tang Y, Zhao CY, Tan ST, Xue HW (2016) Arabidopsis type II Phosphatidylinositol 4-Kinase PI4Kγ5 regulates auxin biosynthesis and leaf margin development through interacting with membrane-bound transcription factor ANAC078. PLoS Genetics, 12, e1006252. |
| [59] | Tao Q, Guo D, Wei B, Zhang F, Pang C, Jiang H, Zhang J, Wei T, Gu H, Qu LJ, Qin G (2013) The TIE1 transcriptional repressor links TCP transcription factors with TOPLESS/ TOPLESS-RELATED corepressors and modulates leaf development in Arabidopsis. The Plant Cell, 25, 421-437. |
| [60] | Vandenbussche F, Petrasek J, Zadnikova P, Hoyerova K, Pesek B, Raz V, Swarup R, Bennett M, Zazimalova E, Benkova E, Van Der Straeten D (2010) The auxin influx carriers AUX1 and LAX3 are involved in auxin-ethylene interactions during apical hook development in Arabidopsis thaliana seedlings. Development, 137, 597-606. |
| [61] | Vlad D, Kierzkowski D, Rast MI, Vuolo F, Ioio RD, Galinha C, Gan X, Hajheidari M, Hay A, Smith RS, Huijser P, Bailey CD, Tsiantis M (2014) Leaf shape evolution through duplication, regulatory diversification, and loss of a homeobox gene. Science, 343, 780-783. |
| [62] | Vollbreeht E, Veit B, Sinha N, Hake S (1991) The developmental gene Knotted-1 is a member of a maize homeobox gene family. Nature, 350, 241-243. |
| [63] | Vroemen CW, Mordhorst AP, Albrecht C, Kwaaitaal MACJ, de Vries SC (2003) The CUP-SHAPED COTYLEDON3 gene is required for boundary and shoot meristem formation in Arabidopsis. The Plant Cell, 15, 1563-1577. |
| [64] | Wolfe JA (1995) Paleoclimatic estimates from tertiary leaf assemblages. Annual Review of Earth Planetary Sciences, 23, 119-142. |
| [65] | Wang Q, Hasson A, Rossmann S, Theres K (2015) Divide et impera: Boundaries shape the plant body and initiate new meristems. New Phytologist, 209, 485-498. |
| [66] | Zhang J, Wei B, Yuan R, Wang J, Ding M, Chen Z, Yu H, Qin G (2017) The Arabidopsis RING-type E3 ligase TEAR1 controls leaf development by targeting the TIE1 transcriptional repressor for degradation. The Plant Cell, 29, 243-259. |
| [1] | Xue Zhang, Yurui Wang, Yangbo Fan, Xiaotian Luo, Xiaozhong Hu, Feng Gao. Morphology, ontogeny and molecular phylogeny of Euplotes aediculatus Pierson, 1943 (Ciliophora, Euplotida) [J]. Biodiv Sci, 2017, 25(5): 549-560. |
| Viewed | ||||||
|
Full text |
|
|||||
|
Abstract |
|
|||||
Copyright © 2022 Biodiversity Science
Editorial Office of Biodiversity Science, 20 Nanxincun, Xiangshan, Beijing 100093, China
Tel: 010-62836137, 62836665 E-mail: biodiversity@ibcas.ac.cn ![]()